Influenza virus vaccine based on the conserved hemagglutinin stalk domain
- PMID: 20689752
- PMCID: PMC2912658
- DOI: 10.1128/mBio.00018-10
Influenza virus vaccine based on the conserved hemagglutinin stalk domain
Abstract
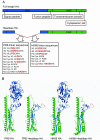
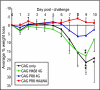
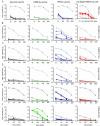
Although highly effective in the general population when well matched to circulating influenza virus strains, current influenza vaccines are limited in their utility due to the narrow breadth of protection they provide. The strain specificity of vaccines presently in use mirrors the exquisite specificity of the neutralizing antibodies that they induce, that is, antibodies which bind to the highly variable globular head domain of hemagglutinin (HA). Herein, we describe the construction of a novel immunogen comprising the conserved influenza HA stalk domain and lacking the globular head. Vaccination of mice with this headless HA construct elicited immune sera with broader reactivity than those obtained from mice immunized with a full-length HA. Furthermore, the headless HA vaccine provided full protection against death and partial protection against disease following lethal viral challenge. Our results suggest that the response induced by headless HA vaccines is sufficiently potent to warrant their further development toward a universal influenza virus vaccine.
Figures
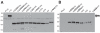


References
-
- Fox J. P., Cooney M. K., Hall C. E., Foy H. M. 1982. Influenza virus infections in Seattle families, 1975-1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am. J. Epidemiol. 116:228–242 - PubMed
-
- Glezen W. P., Couch R. B. 1978. Interpandemic influenza in the Houston area, 1974-1976. N. Engl. J. Med. 298:587–592 - PubMed
-
- Belshe R. B. 2007. Translational research on vaccines: influenza as an example. Clin. Pharmacol. Ther. 82:745–749 - PubMed
-
- Thompson W. W., Shay D. K., Weintraub E., Brammer L., Cox N., Anderson L. J., Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186 - PubMed
-
- Johnson N. P., Mueller J. 2002. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull. Hist. Med. 76:105–115 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
