Differential Antitumor Effects of IgG and IgM Monoclonal Antibodies and Their Synthetic Complementarity-Determining Regions Directed to New Targets of B16F10-Nex2 Melanoma Cells
- PMID: 20689762
- PMCID: PMC2915412
- DOI: 10.1593/tlo.09316
Differential Antitumor Effects of IgG and IgM Monoclonal Antibodies and Their Synthetic Complementarity-Determining Regions Directed to New Targets of B16F10-Nex2 Melanoma Cells
Abstract
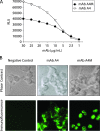
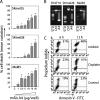
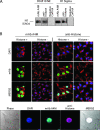
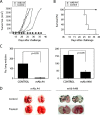
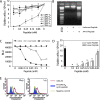
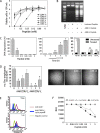
Malignant melanoma has increased incidence worldwide and causes most skin cancer-related deaths. A few cell surface antigens that can be targets of antitumor immunotherapy have been characterized in melanoma. This is an expanding field because of the ineffectiveness of conventional cancer therapy for the metastatic form of melanoma. In the present work, antimelanoma monoclonal antibodies (mAbs) were raised against B16F10 cells (subclone Nex4, grown in murine serum), with novel specificities and antitumor effects in vitro and in vivo. MAb A4 (IgG2ak) recognizes a surface antigen on B16F10-Nex2 cells identified as protocadherin beta(13). It is cytotoxic in vitro and in vivo to B16F10-Nex2 cells as well as in vitro to human melanoma cell lines. MAb A4M (IgM) strongly reacted with nuclei of permeabilized murine tumor cells, recognizing histone 1. Although it is not cytotoxic in vitro, similarly with mAb A4, mAb A4M significantly reduced the number of lung nodules in mice challenged intravenously with B16F10-Nex2 cells. The V(H) CDR3 peptide from mAb A4 and V(L) CDR1 and CDR2 from mAb A4M showed significant cytotoxic activities in vitro, leading tumor cells to apoptosis. A cyclic peptide representing A4 CDR H3 competed with mAb A4 for binding to melanoma cells. MAb A4M CDRs L1 and L2 in addition to the antitumor effect also inhibited angiogenesis of human umbilical vein endothelial cells in vitro. As shown in the present work, mAbs A4 and A4M and selected CDR peptides are strong candidates to be developed as drugs for antitumor therapy for invasive melanoma.
Figures

Similar articles
-
Anti-tumor activities of peptides corresponding to conserved complementary determining regions from different immunoglobulins.Peptides. 2014 Sep;59:14-9. doi: 10.1016/j.peptides.2014.06.007. Epub 2014 Jun 24. Peptides. 2014. PMID: 24972300
-
β-Actin-binding complementarity-determining region 2 of variable heavy chain from monoclonal antibody C7 induces apoptosis in several human tumor cells and is protective against metastatic melanoma.J Biol Chem. 2012 Apr 27;287(18):14912-22. doi: 10.1074/jbc.M111.322362. Epub 2012 Feb 13. J Biol Chem. 2012. PMID: 22334655 Free PMC article.
-
Antibody complementarity-determining regions (CDRs) can display differential antimicrobial, antiviral and antitumor activities.PLoS One. 2008 Jun 11;3(6):e2371. doi: 10.1371/journal.pone.0002371. PLoS One. 2008. PMID: 18545659 Free PMC article.
-
Augmentation of tumor antigen expression by recombinant human interferons: enhanced targeting of monoclonal antibodies to carcinomas.Cancer Treat Res. 1990;51:413-32. doi: 10.1007/978-1-4613-1497-4_21. Cancer Treat Res. 1990. PMID: 1977458 Review.
-
Immunotherapy with monoclonal antibodies in metastatic melanoma.World J Surg. 1992 Mar-Apr;16(2):261-9. doi: 10.1007/BF02071530. World J Surg. 1992. PMID: 1561808 Review.
Cited by
-
Blockade of MIF-CD74 Signalling on Macrophages and Dendritic Cells Restores the Antitumour Immune Response Against Metastatic Melanoma.Front Immunol. 2018 May 23;9:1132. doi: 10.3389/fimmu.2018.01132. eCollection 2018. Front Immunol. 2018. PMID: 29875777 Free PMC article.
-
Antibodies as an unlimited source of anti-infective, anti-tumour and immunomodulatory peptides.Sci Prog. 2014;97(Pt 3):215-33. doi: 10.3184/003685014X14049273183515. Sci Prog. 2014. PMID: 25549407 Free PMC article. Review.
-
A limitless Brazilian scientist: Professor Travassos and his contribution to cancer biology.Braz J Microbiol. 2023 Dec;54(4):2551-2560. doi: 10.1007/s42770-023-01085-0. Epub 2023 Aug 17. Braz J Microbiol. 2023. PMID: 37589929 Free PMC article. Review.
-
Contribution of the Commensal Microflora to the Immunological Homeostasis and the Importance of Immune-Related Drug Development for Clinical Applications.Int J Mol Sci. 2021 Aug 18;22(16):8896. doi: 10.3390/ijms22168896. Int J Mol Sci. 2021. PMID: 34445599 Free PMC article. Review.
-
From antimicrobial to anticancer: the pioneering works of Prof. Luiz Rodolpho Travassos on bioactive peptides.Braz J Microbiol. 2023 Dec;54(4):2561-2570. doi: 10.1007/s42770-023-01118-8. Epub 2023 Sep 19. Braz J Microbiol. 2023. PMID: 37725261 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Terheyden P, Tilgen W, Hauschild A. Recent aspects of medical care of malignant melanoma. J Dtsch Dermatol Ges. 2008;6:868–878. - PubMed
-
- Komenaka I, Hoerig H, Kaufman HL. Immunotherapy for melanoma. Clin Dermatol. 2004;22:251–265. - PubMed
-
- Vulink A, Radford KJ, Melief C, Hart DN. Dendritic cells in cancer immunotherapy. Adv Cancer Res. 2008;99:363–407. - PubMed
-
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547–564. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

