Anti-Angiogenic/Vascular Effects of the mTOR Inhibitor Everolimus Are Not Detectable by FDG/FLT-PET
- PMID: 20689768
- PMCID: PMC2915418
- DOI: 10.1593/tlo.10127
Anti-Angiogenic/Vascular Effects of the mTOR Inhibitor Everolimus Are Not Detectable by FDG/FLT-PET
Abstract
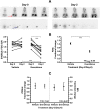
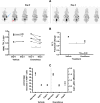
Noninvasive functional imaging of tumors can provide valuable early-response biomarkers, in particular, for targeted chemotherapy. Using various experimental tumor models, we have investigated the ability of positron emission tomography (PET) measurements of 2-deoxy-2-[(18)F]fluoro-glucose (FDG) and 3'-deoxy-3'-[(18)F]fluorothymidine (FLT) to detect response to the allosteric mammalian target of rapamycin (mTOR) inhibitor everolimus. Tumor models were declared sensitive (murine melanoma B16/BL6 and human lung H596) or relatively insensitive (human colon HCT116 and cervical KB31), according to the IC(50) values (concentration inhibiting cell growth by 50%) for inhibition of proliferation in vitro (<10 nM and >1 microM, respectively). Everolimus strongly inhibited growth of the sensitive models in vivo but also significantly inhibited growth of the insensitive models, an effect attributable to its known anti-angiogenic/vascular properties. However, although tumor FDG and FLT uptake was significantly reduced in the sensitive models, it was not affected in the insensitive models, suggesting that endothelial-directed effects could not be detected by these PET tracers. Consistent with this hypothesis, in a well-vascularized orthotopic rat mammary tumor model, other antiangiogenic agents also failed to affect FDG uptake, despite inhibiting tumor growth. In contrast, the cytotoxic patupilone, a microtubule stabilizer, blocked tumor growth, and markedly reduced FDG uptake. These results suggest that FDG/FLT-PET may not be a suitable method for early markers of response to antiangiogenic agents and mTOR inhibitors in which anti-angiogenic/vascular effects predominate because the method could provide false-negative responses. These conclusions warrant clinical testing.
Figures






References
-
- Bjornsti M-A, Houghton PJ. The TOR pathway: a target for cancer chemotherapy. Nat Rev Cancer. 2004;4:335–348. - PubMed
-
- Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, Littlewood-Evans A, Maira SM, Martiny-Baron G, Schnell CR, et al. The mTOR inhibitor RAD001 (everolimus) has anti-angiogenic/vascular properties distinct from a VEGF-R tyrosine kinase inhibitor. Clin Cancer Res. 2009;15:1612–1622. - PubMed
-
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. - PubMed
-
- Korhonen P, Malangone E, Sherman S, Casciano R, Motzer R, Baladi JF, Haas T, Zuber E, Kay A, Lebwohl D. Overall survival (OS) of mRCC patients corrected for crossover using inverse probability of censoring weights (IPCW) and rank preserving structural failure time (RPSFT) models: two analyses from the RECORD-1 trial; Paper presented at: Annual Meeting of the American Society of Clinical Oncology; June 4–8, 2010; Chicago, IL. Abstract 4690.
LinkOut - more resources
Full Text Sources
Miscellaneous

