A Polymorphic Variant of AFAP-110 Enhances cSrc Activity
- PMID: 20689769
- PMCID: PMC2915419
- DOI: 10.1593/tlo.10106
A Polymorphic Variant of AFAP-110 Enhances cSrc Activity
Abstract
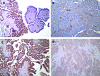
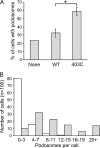
Enhanced expression and activity of cSrc are associated with ovarian cancer progression. Generally, cSrc does not contain activating mutations; rather, its activity is increased in response to signals that affect a conformational change that releases its autoinhibition. In this report, we analyzed ovarian cancer tissues for the expression of a cSrc-activating protein, AFAP-110. AFAP-110 activates cSrc through a direct interaction that releases it from its autoinhibited conformation. Immunohistochemical analysis revealed a concomitant increase of AFAP-110 and cSrc in ovarian cancer tissues. An analysis of the AFAP-110 coding sequence revealed the presence of a nonsynonymous, single-nucleotide polymorphism that resulted in a change of Ser403 to Cys403. In cells that express enhanced levels of cSrc, AFAP-110(403C) directed the activation of cSrc and the formation of podosomes independently of input signals, in contrast to wild-type AFAP-110. We therefore propose that, under conditions of cSrc overexpression, the polymorphic variant of AFAP-110 promotes cSrc activation. Further, these data indicate amechanismby which an inherited genetic variation could influence ovarian cancer progression and could be used to predict the response to targeted therapy.
Figures






References
-
- Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–366. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, LaPolla J, Hoffman M, Martino MA, Wakeley K, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. - PubMed
-
- Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–1706. - PubMed
-
- Goldstein DB, Cavalleri GL. Genomics: understanding human diversity. Nature. 2005;437:1241–1242. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

