Measurement of treatment compliance using a medical device for glaucoma patients associated with intraocular pressure control: a survey
- PMID: 20689790
- PMCID: PMC2915860
- DOI: 10.2147/opth.s11799
Measurement of treatment compliance using a medical device for glaucoma patients associated with intraocular pressure control: a survey
Abstract
Objective: To identify and characterize treatment compliance profiles of glaucoma patients and evaluate the association with intraocular pressure (IOP).
Methods: A computerized device (Travalert((R))) that recorded daily instillation times and eye-drop counts was given for 3 months. Patients were declared compliant when at least 2 drops were instilled per day. Compliance rates were calculated for weekdays and weekends, separately, over 8 consecutive weeks. A principal components analysis (PCA) was followed by an ascendant hierarchical classification (AHC) to identify compliance groups.
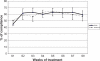
Results: 140 patients were recruited (mean age 65.5 years; 51.8% female) of whom 83.6% had primary open-angle glaucoma with mean IOP 23.9 mmHg before Travalert((R)) use. 60.7% were treated with DuoTrav((R)) (travoprost timolol fixed combination) and 39.3% with travoprost. The PCA identified two axes (compliance and treatment weeks). The AHC identified 3 compliance groups: 'high' (56.6%, approx. 80% compliance), 'medium' (21.2%, approx. 50% compliance), and 'low' (22.1%, approx. 20% compliance). Demographics and glaucoma parameters did not predict low compliance. Final mean IOP was 16.1 mmHg, but higher in the low compliance group (17.7 mmHg, P = 0.02).
Conclusions: Compliance measurement by a medical device showed compliance rates <80% by 50% (approx.) of patients, significantly impacting IOP control. No demographic or glaucoma variable was associated with low compliance.
Keywords: compliance; efficacy; glaucoma; intraocular pressure control.
Figures



Similar articles
-
Fixed Combination of Travoprost and Timolol Maleate Reduces Intraocular Pressure in Japanese Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: A Prospective Multicenter Open-Label Study.Adv Ther. 2015 Sep;32(9):823-37. doi: 10.1007/s12325-015-0246-9. Epub 2015 Sep 30. Adv Ther. 2015. PMID: 26424331 Free PMC article. Clinical Trial.
-
Intraocular pressure control with latanoprost/timolol and travoprost/timolol fixed combinations : a retrospective, multicentre, cross-sectional study.Clin Drug Investig. 2008;28(12):767-76. doi: 10.2165/0044011-200828120-00004. Clin Drug Investig. 2008. PMID: 18991470 Clinical Trial.
-
A three-month, multicenter, double-masked study of the safety and efficacy of travoprost 0.004%/timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma or ocular hypertension.J Glaucoma. 2005 Oct;14(5):392-9. doi: 10.1097/01.ijg.0000176935.08392.14. J Glaucoma. 2005. PMID: 16148589 Clinical Trial.
-
The efficacy of the fixed combination of latanoprost and timolol versus other fixed combinations for primary open-angle glaucoma and ocular hypertension: A systematic review and meta-analysis.PLoS One. 2020 Feb 27;15(2):e0229682. doi: 10.1371/journal.pone.0229682. eCollection 2020. PLoS One. 2020. PMID: 32106236 Free PMC article.
-
Latanoprost : an update of its use in glaucoma and ocular hypertension.Drugs Aging. 2003;20(8):597-630. doi: 10.2165/00002512-200320080-00005. Drugs Aging. 2003. PMID: 12795627 Review.
Cited by
-
Drop instillation and glaucoma.Curr Opin Ophthalmol. 2018 Mar;29(2):171-177. doi: 10.1097/ICU.0000000000000451. Curr Opin Ophthalmol. 2018. PMID: 29140818 Free PMC article. Review.
-
Exploring the influence of patient-provider communication on intraocular pressure in glaucoma patients.Patient Educ Couns. 2015 Jul 6:S0738-3991(15)30010-0 10.1016/j.pec.2015.07.001. doi: 10.1016/j.pec.2015.07.001. Online ahead of print. Patient Educ Couns. 2015. PMID: 26223851 Free PMC article.
-
Impact of a health communication intervention to improve glaucoma treatment adherence. Results of the interactive study to increase glaucoma adherence to treatment trial.Arch Ophthalmol. 2012 Oct;130(10):1252-8. doi: 10.1001/archophthalmol.2012.1607. Arch Ophthalmol. 2012. PMID: 22688429 Free PMC article. Clinical Trial.
-
Identification of noncompliant glaucoma patients using Bayesian networks and the Eye-Drop Satisfaction Questionnaire.Clin Ophthalmol. 2010 Dec 8;4:1489-96. doi: 10.2147/OPTH.S11818. Clin Ophthalmol. 2010. PMID: 21191445 Free PMC article.
-
Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems.Nat Rev Drug Discov. 2023 May;22(5):387-409. doi: 10.1038/s41573-023-00670-0. Epub 2023 Mar 27. Nat Rev Drug Discov. 2023. PMID: 36973491 Free PMC article. Review.
References
-
- Weih LM, Nanjan M, McCarty CA, Taylor HR. Prevalence and predictors of open-angle glaucoma: results from the visual impairment project. Ophthalmology. 2001;108:1966–1972. - PubMed
-
- Mitchell P, Smith W, Attebo K, et al. Prevalence of open-angle glaucoma in Australia. The Blue Mountains eye study. Ophthalmology. 1996;103:1661–1669. - PubMed
-
- Klein BEK, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–1504. - PubMed
-
- Rudnicka AR, Mt-Isa S, Owen CG, et al. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47:4254–4261. - PubMed
-
- Bron A, Baudouin C, Nordmann JP, et al. [Prevalence of intraocular hypertension and glaucoma in a nonselected French population] J Fr Ophtalmol. 2006;29:635–641. French. - PubMed
LinkOut - more resources
Full Text Sources

