Rac1-dependent collective cell migration is required for specification of the anterior-posterior body axis of the mouse
- PMID: 20689803
- PMCID: PMC2914637
- DOI: 10.1371/journal.pbio.1000442
Rac1-dependent collective cell migration is required for specification of the anterior-posterior body axis of the mouse
Abstract
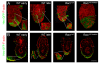
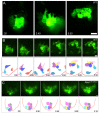
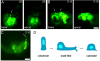
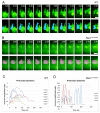
Cell migration and cell rearrangements are critical for establishment of the body plan of vertebrate embryos. The first step in organization of the body plan of the mouse embryo, specification of the anterior-posterior body axis, depends on migration of the anterior visceral endoderm from the distal tip of the embryo to a more proximal region overlying the future head. The anterior visceral endoderm (AVE) is a cluster of extra-embryonic cells that secretes inhibitors of the Wnt and Nodal pathways to inhibit posterior development. Because Rac proteins are crucial regulators of cell migration and mouse Rac1 mutants die early in development, we tested whether Rac1 plays a role in AVE migration. Here we show that Rac1 mutant embryos fail to specify an anterior-posterior axis and, instead, express posterior markers in a ring around the embryonic circumference. Cells that express the molecular markers of the AVE are properly specified in Rac1 mutants but remain at the distal tip of the embryo at the time when migration should take place. Using tissue specific deletions, we show that Rac1 acts autonomously within the visceral endoderm to promote cell migration. High-resolution imaging shows that the leading wild-type AVE cells extend long lamellar protrusions that span several cell diameters and are polarized in the direction of cell movement. These projections are tipped by filopodia-like structures that appear to sample the environment. Wild-type AVE cells display hallmarks of collective cell migration: they retain tight and adherens junctions as they migrate and exchange neighbors within the plane of the visceral endoderm epithelium. Analysis of mutant embryos shows that Rac1 is not required for intercellular signaling, survival, proliferation, or adhesion in the visceral endoderm but is necessary for the ability of visceral endoderm cells to extend projections, change shape, and exchange neighbors. The data show that Rac1-mediated epithelial migration of the AVE is a crucial step in the establishment of the mammalian body plan and suggest that Rac1 is essential for collective migration in mammalian tissues.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
β-Pix directs collective migration of anterior visceral endoderm cells in the early mouse embryo.Genes Dev. 2014 Dec 15;28(24):2764-77. doi: 10.1101/gad.251371.114. Genes Dev. 2014. PMID: 25512563 Free PMC article.
-
Nodal dependent differential localisation of dishevelled-2 demarcates regions of differing cell behaviour in the visceral endoderm.PLoS Biol. 2011 Feb;9(2):e1001019. doi: 10.1371/journal.pbio.1001019. Epub 2011 Feb 22. PLoS Biol. 2011. PMID: 21364967 Free PMC article.
-
Regionalization of the mouse visceral endoderm as the blastocyst transforms into the egg cylinder.BMC Dev Biol. 2007 Aug 16;7:96. doi: 10.1186/1471-213X-7-96. BMC Dev Biol. 2007. PMID: 17705827 Free PMC article.
-
Heading forwards: anterior visceral endoderm migration in patterning the mouse embryo.Philos Trans R Soc Lond B Biol Sci. 2014 Dec 5;369(1657):20130546. doi: 10.1098/rstb.2013.0546. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25349454 Free PMC article. Review.
-
The Head's Tale: Anterior-Posterior Axis Formation in the Mouse Embryo.Curr Top Dev Biol. 2018;128:365-390. doi: 10.1016/bs.ctdb.2017.11.003. Epub 2017 Dec 8. Curr Top Dev Biol. 2018. PMID: 29477169 Review.
Cited by
-
Asymmetry in the frequency and position of mitosis in the mouse embryo epiblast at gastrulation.EMBO Rep. 2020 Nov 5;21(11):e50944. doi: 10.15252/embr.202050944. Epub 2020 Oct 5. EMBO Rep. 2020. PMID: 33016470 Free PMC article.
-
Regulation of extra-embryonic endoderm stem cell differentiation by Nodal and Cripto signaling.Development. 2011 Sep;138(18):3885-95. doi: 10.1242/dev.065656. Development. 2011. PMID: 21862554 Free PMC article.
-
The Dynamics of Morphogenesis in the Early Mouse Embryo.Cold Spring Harb Perspect Biol. 2014 Jun 26;7(11):a015867. doi: 10.1101/cshperspect.a015867. Cold Spring Harb Perspect Biol. 2014. PMID: 24968703 Free PMC article. Review.
-
Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development.Organogenesis. 2016 Oct;12(4):194-216. doi: 10.1080/15476278.2016.1252887. Epub 2016 Nov 14. Organogenesis. 2016. PMID: 27841695 Free PMC article.
-
Origin, fate and function of extraembryonic tissues during mammalian development.Nat Rev Mol Cell Biol. 2025 Apr;26(4):255-275. doi: 10.1038/s41580-024-00809-w. Epub 2024 Dec 3. Nat Rev Mol Cell Biol. 2025. PMID: 39627419 Review.
References
-
- Thomas P. Q, Brown A, Beddington R. S. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125:85–94. - PubMed
-
- Srinivas S, Rodriguez T, Clements M, Smith J. C, Beddington R. S. P. Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development. 2004;131:1157–1164. - PubMed
-
- Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, et al. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Developmental Cell. 2005;9:639–650. - PubMed
-
- Srinivas S. The anterior visceral endoderm-turning heads. Genesis. 2006;44:565–572. - PubMed
-
- Salgueiro A. M, Filipe M, Belo J. A. N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase expression during early mouse embryonic development. Int J Dev Biol. 2006;50:705–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

