The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants
- PMID: 20689824
- PMCID: PMC2914784
- DOI: 10.1371/journal.pone.0011937
The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants
Abstract
Background: The pathogenesis of immunodeficiency due to human immunodeficiency virus (HIV)-1 is incompletely understood, but immune activation is believed to play a central role. Immunomodulatory agents that decrease immune activation may be useful in the treatment of HIV-1 infection.
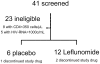
Methodology: A randomized, double blind, placebo-controlled pilot study of leflunomide for 28 days was performed in participants with HIV-1 infection who were not receiving antiretroviral therapy. Participants randomized to leflunomide were subsequently treated with cholestyramine until leflunomide levels were below detection limit.
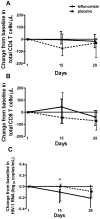
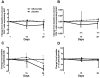
Findings: Treatment with leflunomide was well tolerated with mostly low-grade adverse events. Leflunomide administration reduced cycling of CD4 T cells (by ex vivo bromodeoxyuridine uptake and Ki67 expression) and decreased expression of activation markers (HLA-DR/CD38 co-expression) on CD8 T cells in peripheral blood. In addition, decreased expression of HIV-1 co-receptors was observed in both CD4 and CD8 T cells in the leflunomide group. There were no significant changes in naïve and memory T cell subsets, apoptosis of T cells or markers of microbial translocation.
Conclusions: Leflunomide was effective in reducing immune activation in the setting of chronic HIV-1 infection suggesting that targeting immune activation with immunomodulatory agents may be a feasible strategy.
Trial registration: ClinicalTrials.gov NCT00101374.
Conflict of interest statement
Figures

Similar articles
-
Rapid restoration of CD4 T cell subsets in subjects receiving antiretroviral therapy during primary HIV-1 infection.AIDS. 2000 Dec 1;14(17):2643-51. doi: 10.1097/00002030-200012010-00003. AIDS. 2000. PMID: 11125882 Clinical Trial.
-
[T-lymphocyte immune in HIV-infected people and AIDS patients in China].Zhonghua Yi Xue Za Zhi. 2002 Oct 25;82(20):1391-5. Zhonghua Yi Xue Za Zhi. 2002. PMID: 12509921 Chinese.
-
Combined Env- and Gag-specific T cell responses in relation to programmed death-1 receptor and CD4 T cell loss rates in human immunodeficiency virus-1 infection.Clin Exp Immunol. 2010 Aug;161(2):315-23. doi: 10.1111/j.1365-2249.2010.04179.x. Epub 2010 May 10. Clin Exp Immunol. 2010. PMID: 20491784 Free PMC article.
-
Characterization of CD31 expression in CD4+ and CD8+T cell subpopulations in chronic untreated HIV infection.Immunol Lett. 2021 Jul;235:22-31. doi: 10.1016/j.imlet.2021.04.004. Epub 2021 Apr 20. Immunol Lett. 2021. PMID: 33852965
-
Changes in CD4+ and CD8+ T cell subsets in response to highly active antiretroviral therapy in HIV type 1-infected patients with prior protease inhibitor experience.AIDS Res Hum Retroviruses. 1998 May 1;14(7):561-9. doi: 10.1089/aid.1998.14.561. AIDS Res Hum Retroviruses. 1998. PMID: 9591710
Cited by
-
CD38 Expression in a Subset of Memory T Cells Is Independent of Cell Cycling as a Correlate of HIV Disease Progression.Dis Markers. 2016;2016:9510756. doi: 10.1155/2016/9510756. Epub 2016 Mar 14. Dis Markers. 2016. PMID: 27064238 Free PMC article.
-
Efficacy and safety of dihydroorotate dehydrogenase (DHODH) inhibitors "leflunomide" and "teriflunomide" in Covid-19: A narrative review.Eur J Pharmacol. 2021 Sep 5;906:174233. doi: 10.1016/j.ejphar.2021.174233. Epub 2021 Jun 7. Eur J Pharmacol. 2021. PMID: 34111397 Free PMC article. Review.
-
Residual immune dysfunction under antiretroviral therapy.Semin Immunol. 2021 Jan;51:101471. doi: 10.1016/j.smim.2021.101471. Epub 2021 Mar 3. Semin Immunol. 2021. PMID: 33674177 Free PMC article. Review.
-
HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies.J Immunol Res. 2021 Sep 29;2021:7316456. doi: 10.1155/2021/7316456. eCollection 2021. J Immunol Res. 2021. PMID: 34631899 Free PMC article. Review.
-
HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo.J Virol. 2011 Oct;85(19):10189-200. doi: 10.1128/JVI.02529-10. Epub 2011 Aug 3. J Virol. 2011. PMID: 21813616 Free PMC article.
References
-
- Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, et al. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:332–340. - PubMed
-
- Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. - PubMed
-
- Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. Aids. 2003;17:1881–1888. - PubMed
-
- Anthony KB, Yoder C, Metcalf JA, DerSimonian R, Orenstein JM, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33:125–133. - PubMed
-
- Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

