Neuronal PINCH is regulated by TNF-α and is required for neurite extension
- PMID: 20689998
- PMCID: PMC3107369
- DOI: 10.1007/s11481-010-9236-5
Neuronal PINCH is regulated by TNF-α and is required for neurite extension
Abstract
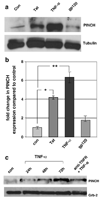
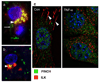
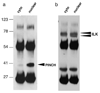
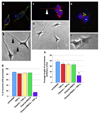
During HIV infection of the CNS, neurons are damaged by viral proteins, such as Tat and gp120, or by inflammatory factors, such as TNF-α, that are released from infected and/or activated glial cells. Host responses to this damage may include the induction of survival or repair mechanisms. In this context, previous studies report robust expression of a protein called particularly interesting new cysteine histidine-rich protein (PINCH), in the neurons of HIV patients' brains, compared with nearly undetectable levels in HIV-negative individuals (Rearden et al., J Neurosci Res 86:2535-2542, 2008), suggesting PINCH's involvement in neuronal signaling during HIV infection of the brain. To address potential triggers for PINCH induction in HIV patients' brains, an in vitro system mimicking some aspects of HIV infection of the CNS was utilized. We investigated neuronal PINCH expression, subcellular distribution, and biological consequences of PINCH sequestration upon challenge with Tat, gp120, and TNF-α. Our results indicate that in neurons, TNF-α stimulation increases PINCH expression and changes its subcellular localization. Furthermore, PINCH mobility is required to maintain neurite extension upon challenge with TNF-α. PINCH may function as a neuron-specific host-mediated response to challenge by HIV-related factors in the CNS.
Figures


Similar articles
-
Inflammation-induced PINCH expression leads to actin depolymerization and mitochondrial mislocalization in neurons.Transl Neurodegener. 2020 Aug 3;9(1):32. doi: 10.1186/s40035-020-00211-4. Transl Neurodegener. 2020. PMID: 32746944 Free PMC article.
-
PINCH: More than just an adaptor protein in cellular response.J Cell Physiol. 2011 Apr;226(4):940-7. doi: 10.1002/jcp.22437. J Cell Physiol. 2011. PMID: 20945343 Free PMC article. Review.
-
Novel expression of PINCH in the central nervous system and its potential as a biomarker for human immunodeficiency virus-associated neurodegeneration.J Neurosci Res. 2008 Aug 15;86(11):2535-42. doi: 10.1002/jnr.21701. J Neurosci Res. 2008. PMID: 18459134 Free PMC article.
-
Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia.J Neurovirol. 1998 Jun;4(3):281-90. doi: 10.3109/13550289809114529. J Neurovirol. 1998. PMID: 9639071
-
Tumor necrosis factor-alpha at the crossroads of neuronal life and death during HIV-associated dementia.J Neurochem. 2003 Sep;86(5):1057-71. doi: 10.1046/j.1471-4159.2003.01942.x. J Neurochem. 2003. PMID: 12911614 Free PMC article. Review.
Cited by
-
Integrin-Linked Kinase (ILK) Plays an Important Role in the Laminin-Dependent Development of Dorsal Root Ganglia during Chicken Embryogenesis.Cells. 2021 Jul 2;10(7):1666. doi: 10.3390/cells10071666. Cells. 2021. PMID: 34359835 Free PMC article.
-
Inflammation-induced PINCH expression leads to actin depolymerization and mitochondrial mislocalization in neurons.Transl Neurodegener. 2020 Aug 3;9(1):32. doi: 10.1186/s40035-020-00211-4. Transl Neurodegener. 2020. PMID: 32746944 Free PMC article.
-
Cocaine-mediated activation of microglia and microglial MeCP2 and BDNF production.Neurobiol Dis. 2018 Sep;117:28-41. doi: 10.1016/j.nbd.2018.05.017. Epub 2018 May 30. Neurobiol Dis. 2018. PMID: 29859319 Free PMC article.
-
Signaling via PINCH: Functions, binding partners and implications in human diseases.Gene. 2016 Dec 5;594(1):10-15. doi: 10.1016/j.gene.2016.08.039. Epub 2016 Aug 30. Gene. 2016. PMID: 27590440 Free PMC article. Review.
-
PINCH: More than just an adaptor protein in cellular response.J Cell Physiol. 2011 Apr;226(4):940-7. doi: 10.1002/jcp.22437. J Cell Physiol. 2011. PMID: 20945343 Free PMC article. Review.
References
-
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25:123–133. - PubMed
-
- Aukrust P, Liabakk NB, Muller F, Lien E, Espevik T, Froland SS. Serum levels of tumor necrosis factor-alpha (TNF alpha) and soluble TNF receptors in human immunodeficiency virus type 1 infection—correlations to clinical, immunologic, and virologic parameters. J Infect Dis. 1994;169:420–424. - PubMed
-
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

