Assembly of fibronectin extracellular matrix
- PMID: 20690820
- PMCID: PMC3628685
- DOI: 10.1146/annurev-cellbio-100109-104020
Assembly of fibronectin extracellular matrix
Abstract
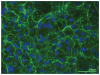
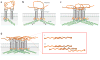
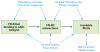
In the process of matrix assembly, multivalent extracellular matrix (ECM) proteins are induced to self-associate and to interact with other ECM proteins to form fibrillar networks. Matrix assembly is usually initiated by ECM glycoproteins binding to cell surface receptors, such as fibronectin (FN) dimers binding to α5ß1 integrin. Receptor binding stimulates FN self-association mediated by the N-terminal assembly domain and organizes the actin cytoskeleton to promote cell contractility. FN conformational changes expose additional binding sites that participate in fibril formation and in conversion of fibrils into a stabilized, insoluble form. Once assembled, the FN matrix impacts tissue organization by contributing to the assembly of other ECM proteins. Here, we describe the major steps, molecular interactions, and cellular mechanisms involved in assembling FN dimers into fibrillar matrix while highlighting important issues and major questions that require further investigation.
Figures


References
-
- Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem. 1994;269:27863–68. - PubMed
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Science; 2008. p. 1268.
-
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–65. - PubMed
-
- Aota S, Nomizu M, Yamada K. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell adhesive function. J Biol Chem. 1994;269:24756–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

