Involvement of the Reck tumor suppressor protein in maternal and embryonic vascular remodeling in mice
- PMID: 20691046
- PMCID: PMC2923112
- DOI: 10.1186/1471-213X-10-84
Involvement of the Reck tumor suppressor protein in maternal and embryonic vascular remodeling in mice
Abstract
Background: Developmental angiogenesis proceeds through multiple morphogenetic events including sprouting, intussusception, and pruning. Mice lacking the membrane-anchored metalloproteinase regulator Reck die in utero around embryonic day 10.5 with halted vascular development; however, the mechanisms by which this phenotype arises remain unclear.
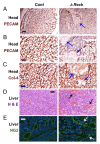
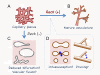
Results: We found that Reck is abundantly expressed in the cells associated with blood vessels undergoing angiogenesis or remodelling in the uteri of pregnant female mice. Some of the Reck-positive vessels show morphological features consistent with non-sprouting angiogenesis. Treatment with a vector expressing a small hairpin RNA against Reck severely disrupts the formation of blood vessels with a compact, round lumen. Similar defects were found in the vasculature of Reck-deficient or Reck conditional knockout embryos.
Conclusions: Our findings implicate Reck in vascular remodeling, possibly through non-sprouting angiogenesis, in both maternal and embyonic tissues.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

