Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome
- PMID: 20691405
- PMCID: PMC2917716
- DOI: 10.1016/j.ajhg.2010.07.003
Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome
Abstract
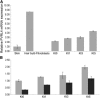
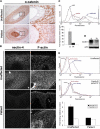
Ectodermal dysplasias form a large disease family with more than 200 members. The combination of hair and tooth abnormalities, alopecia, and cutaneous syndactyly is characteristic of ectodermal dysplasia-syndactyly syndrome (EDSS). We used a homozygosity mapping approach to map the EDSS locus to 1q23 in a consanguineous Algerian family. By candidate gene analysis, we identified a homozygous mutation in the PVRL4 gene that not only evoked an amino acid change but also led to exon skipping. In an Italian family with two siblings affected by EDSS, we further detected a missense and a frameshift mutation. PVRL4 encodes for nectin-4, a cell adhesion molecule mainly implicated in the formation of cadherin-based adherens junctions. We demonstrated high nectin-4 expression in hair follicle structures, as well as in the separating digits of murine embryos, the tissues mainly affected by the EDSS phenotype. In patient keratinocytes, mutated nectin-4 lost its capability to bind nectin-1. Additionally, in discrete structures of the hair follicle, we found alterations of the membrane localization of nectin-afadin and cadherin-catenin complexes, which are essential for adherens junction formation, and we found reorganization of actin cytoskeleton. Together with cleft lip and/or palate ectodermal dysplasia (CLPED1, or Zlotogora-Ogur syndrome) due to an impaired function of nectin-1, EDSS is the second known "nectinopathy" caused by mutations in a nectin adhesion molecule.
Figures



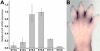
References
-
- Gumbiner B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. - PubMed
-
- Perez-Moreno M., Jamora C., Fuchs E. Sticky business: Orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. - PubMed
-
- Freire-Maia N. Ectodermal dysplasias. Hum. Hered. 1971;21:309–312. - PubMed
-
- Freire-Maia N., Lisboa-Costa T., Pagnan N.A. Ectodermal dysplasias: How many? Am. J. Med. Genet. 2001;104:84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

