The role of L1 stalk-tRNA interaction in the ribosome elongation cycle
- PMID: 20691699
- PMCID: PMC2967302
- DOI: 10.1016/j.jmb.2010.07.056
The role of L1 stalk-tRNA interaction in the ribosome elongation cycle
Abstract
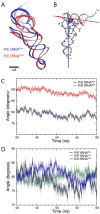
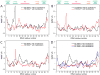
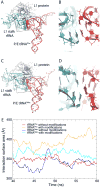
The ribosomal L1 stalk is a mobile structure implicated in directing tRNA movement during translocation through the ribosome. This article investigates three aspects of L1 stalk-tRNA interaction. First, by combining data from cryo electron microscopy, X-ray crystallography, and molecular dynamics simulations through the molecular dynamics flexible fitting method, we obtained atomic models of different tRNAs occupying the hybrid P/E state interacting with the L1 stalk. These models confirm the assignment of fluorescence resonance energy transfer states from previous single-molecule investigations of L1 stalk dynamics. Second, the models reconcile how initiator tRNA(fMet) interacts less strongly with the L1 stalk compared to elongator tRNA(Phe), as seen in previous single-molecule experiments. Third, results from a simulation of the entire ribosome in which the L1 stalk is moved from a half-closed conformation to its open conformation are found to support the hypothesis that L1 stalk opening is involved in tRNA release from the ribosome.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures



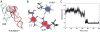

Similar articles
-
Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation.Mol Cell. 2008 May 9;30(3):348-59. doi: 10.1016/j.molcel.2008.03.012. Mol Cell. 2008. PMID: 18471980
-
Initiation factor 2 stabilizes the ribosome in a semirotated conformation.Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):15874-9. doi: 10.1073/pnas.1520337112. Epub 2015 Dec 14. Proc Natl Acad Sci U S A. 2015. PMID: 26668356 Free PMC article.
-
Allosteric collaboration between elongation factor G and the ribosomal L1 stalk directs tRNA movements during translation.Proc Natl Acad Sci U S A. 2009 Sep 15;106(37):15702-7. doi: 10.1073/pnas.0908077106. Epub 2009 Aug 27. Proc Natl Acad Sci U S A. 2009. PMID: 19717422 Free PMC article.
-
Molecular simulations of the ribosome and associated translation factors.Curr Opin Struct Biol. 2018 Apr;49:27-35. doi: 10.1016/j.sbi.2017.11.003. Epub 2017 Dec 1. Curr Opin Struct Biol. 2018. PMID: 29202442 Review.
-
Interaction of tRNA with eukaryotic ribosome.Int J Mol Sci. 2015 Mar 30;16(4):7173-94. doi: 10.3390/ijms16047173. Int J Mol Sci. 2015. PMID: 25830484 Free PMC article. Review.
Cited by
-
Structural insights into mammalian mitochondrial translation elongation catalyzed by mtEFG1.EMBO J. 2020 Aug 3;39(15):e104820. doi: 10.15252/embj.2020104820. Epub 2020 Jun 30. EMBO J. 2020. PMID: 32602580 Free PMC article.
-
Cryo-electron microscopy modeling by the molecular dynamics flexible fitting method.Biopolymers. 2012 Sep;97(9):678-86. doi: 10.1002/bip.22042. Biopolymers. 2012. PMID: 22696404 Free PMC article.
-
Ribosome assembly factors prevent premature translation initiation by 40S assembly intermediates.Science. 2011 Sep 9;333(6048):1449-53. doi: 10.1126/science.1208245. Epub 2011 Aug 11. Science. 2011. PMID: 21835981 Free PMC article.
-
Parallel Generalized Born Implicit Solvent Calculations with NAMD.J Chem Theory Comput. 2011 Nov 8;7(11):3635-3642. doi: 10.1021/ct200563j. J Chem Theory Comput. 2011. PMID: 22121340 Free PMC article.
-
Single-molecule nanometry for biological physics.Rep Prog Phys. 2013 Jan;76(1):016601. doi: 10.1088/0034-4885/76/1/016601. Epub 2012 Dec 18. Rep Prog Phys. 2013. PMID: 23249673 Free PMC article. Review.
References
-
- Moazed D, Noller H. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989;342:142–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

