Lung organogenesis
- PMID: 20691848
- PMCID: PMC3340128
- DOI: 10.1016/S0070-2153(10)90003-3
Lung organogenesis
Abstract
Developmental lung biology is a field that has the potential for significant human impact: lung disease at the extremes of age continues to cause major morbidity and mortality worldwide. Understanding how the lung develops holds the promise that investigators can use this knowledge to aid lung repair and regeneration. In the decade since the "molecular embryology" of the lung was first comprehensively reviewed, new challenges have emerged-and it is on these that we focus the current review. Firstly, there is a critical need to understand the progenitor cell biology of the lung in order to exploit the potential of stem cells for the treatment of lung disease. Secondly, the current familiar descriptions of lung morphogenesis governed by growth and transcription factors need to be elaborated upon with the reinclusion and reconsideration of other factors, such as mechanics, in lung growth. Thirdly, efforts to parse the finer detail of lung bud signaling may need to be combined with broader consideration of overarching mechanisms that may be therapeutically easier to target: in this arena, we advance the proposal that looking at the lung in general (and branching in particular) in terms of clocks may yield unexpected benefits.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
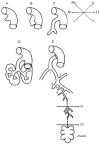


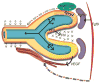
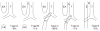

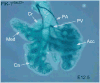

References
-
- Acosta JM, Thebaud B, Castillo C, Mailleux A, Tefft D, Wuenschell C, Anderson KD, Bourbon J, Thiery JP, Bellusci S, Warburton D. Novel mechanisms in murine nitrofen-induced pulmonary hypoplasia: FGF-10 rescue in culture. Am J Physiol Lung Cell Mol Physiol. 2001;281:L250–257. - PubMed
-
- Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

