Building pathways for ovary organogenesis in the mouse embryo
- PMID: 20691852
- PMCID: PMC3400115
- DOI: 10.1016/S0070-2153(10)90007-0
Building pathways for ovary organogenesis in the mouse embryo
Abstract
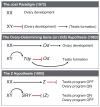
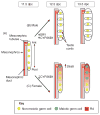
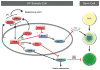
Despite its significant role in oocyte generation and hormone production in adulthood, the ovary, with regard to its formation, has received little attention compared to its male counterpart, the testis. With the exception of germ cells, which undergo a female-specific pattern of meiosis, morphological changes in the fetal ovary are subtle. Over the past 40 years, a number of hypotheses have been proposed for the organogenesis of the mammalian ovary. It was not until the turn of the millennium, thanks to the advancement of genetic and genomic approaches, that pathways for ovary organogenesis that consist of positive and negative regulators have started to emerge. Through the action of secreted factors (R-spondin1, WNT4, and follistatin) and transcription regulators (beta-catenin and FOXL2), the developmental fate of the somatic cells is directed toward ovarian, while testicular components are suppressed. In this chapter, we review the history of studying ovary organogenesis in mammals and present the most recent discoveries using the mouse as the model organism.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. - PubMed
-
- Anderson R, Copeland TK, Scholer H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91:61–68. - PubMed
-
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. - PubMed
-
- Barbaro M, Cicognani A, Balsamo A, Lofgren A, Baldazzi L, Wedell A, Oscarson M. Gene dosage imbalances in patients with 46, XY gonadal DSD detected by an in-house-designed synthetic probe set for multiplex ligation-dependent probe amplification analysis. Clin Genet. 2008;73:453–464. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

