Myc-nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation
- PMID: 20691906
- PMCID: PMC2923036
- DOI: 10.1016/j.cell.2010.06.037
Myc-nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation
Abstract
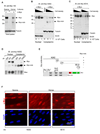
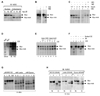
The Myc oncoprotein family comprises transcription factors that control multiple cellular functions and are widely involved in oncogenesis. Here we report the identification of Myc-nick, a cytoplasmic form of Myc generated by calpain-dependent proteolysis at lysine 298 of full-length Myc. Myc-nick retains conserved Myc box regions but lacks nuclear localization signals and the bHLHZ domain essential for heterodimerization with Max and DNA binding. Myc-nick induces alpha-tubulin acetylation and altered cell morphology by recruiting histone acetyltransferase GCN5 to microtubules. During muscle differentiation, while the levels of full-length Myc diminish, Myc-nick and acetylated alpha-tubulin levels are increased. Ectopic expression of Myc-nick accelerates myoblast fusion, triggers the expression of myogenic markers, and permits Myc-deficient fibroblasts to transdifferentiate in response to MyoD. We propose that the cleavage of Myc by calpain abrogates the transcriptional inhibition of differentiation by full-length Myc and generates Myc-nick, a driver of cytoplasmic reorganization and differentiation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures





References
-
- Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. - PubMed
-
- Bai MK, Costopoulos JS, Christoforidou BP, Papadimitriou CS. Immunohistochemical detection of the c-myc oncogene product in normal, hyperplastic and carcinomatous endometrium. Oncology. 1994;51:314–319. - PubMed
-
- Calcagno DQ, Guimaraes AC, Leal MF, Seabra AD, Khayat AS, Pontes TB, Assumpcao PP, De Arruda Cardoso Smith M, Burbano RR. MYC insertions in diffuse-type gastric adenocarcinoma. Anticancer Res. 2009;29:2479–2483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

