Lessons for cardiac regeneration and repair through development
- PMID: 20692205
- PMCID: PMC3089764
- DOI: 10.1016/j.molmed.2010.06.003
Lessons for cardiac regeneration and repair through development
Abstract
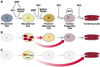
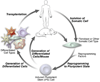
Cell-based regenerative strategies have the potential to revolutionize the way cardiovascular injury is treated, but successful therapies will require a precise understanding of the mechanisms that dictate cell fate, survival and differentiation. Recent advances in the study of cardiac development hold promise for unlocking the keys for successful therapies. Using mouse models and embryonic stem cells, researchers are uncovering cardiac progenitor cells in both embryonic and adult contexts. Furthermore, the signaling molecules and transcriptional regulators that govern these cells and their behavior are being revealed. Here, we focus on the recent advances in these areas of cardiac developmental research and their impact on the expanding field of regenerative medicine.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

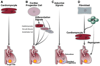
References
-
- Lloyd-Jones D, et al. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2009 - PubMed
-
- Murry CE, et al. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112:3174–3183. - PubMed
-
- Hansson EM, et al. Regeneration Next: Toward Heart Stem Cell Therapeutics. Cell Stem Cell. 2009;5:364–377. - PubMed
-
- Laflamme MA, Murry CE. Regenerating the heart. Nature Biotechnology. 2005;23:845–856. - PubMed
-
- Alvarez-Dolado M, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

