Defective adult oligodendrocyte and Schwann cell development, pigment pattern, and craniofacial morphology in puma mutant zebrafish having an alpha tubulin mutation
- PMID: 20692250
- PMCID: PMC2957186
- DOI: 10.1016/j.ydbio.2010.07.035
Defective adult oligodendrocyte and Schwann cell development, pigment pattern, and craniofacial morphology in puma mutant zebrafish having an alpha tubulin mutation
Abstract
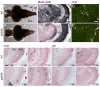
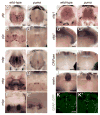
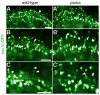
The processes of myelination remain incompletely understood but are of profound biomedical importance owing to the several dysmyelinating and demyelinating disorders known in humans. Here, we analyze the zebrafish puma mutant, isolated originally for pigment pattern defects limited to the adult stage. We show that puma mutants also have late-arising defects in Schwann cells of the peripheral nervous system, locomotor abnormalities, and sex-biased defects in adult craniofacial morphology. Using methods of positional cloning, we identify a critical genetic interval harboring two alpha tubulin loci, and we identify a chemically induced missense mutation in one of these, tubulin alpha 8-like 3a (tuba8l3a). We demonstrate tuba8l3a expression in the central nervous system (CNS), leading us to search for defects in the development of oligodendrocytes, the myelinating cells of the CNS. We find gross reductions in CNS myelin and oligodendrocyte numbers in adult puma mutants, and these deficits are apparent already during the larval-to-adult transformation. By contrast, analyses of embryos and early larvae reveal a normal complement of oligodendrocytes that nevertheless fail to localize normal amounts of myelin basic protein (mbp) mRNA in cellular processes, and fail to organize these processes as in the wild-type. This study identifies the puma mutant as a valuable model for studying microtubule-dependent events of myelination, as well as strategies for remyelination in the adult.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures







References
-
- Aspengren S, Hedberg D, Skold HN, Wallin M. New insights into melanosome transport in vertebrate pigment cells. Int Rev Cell Mol Biol. 2009;272:245–302. - PubMed
-
- Barbarese E, Brumwell C, Kwon S, Cui H, Carson JH. RNA on the road to myelin. J Neurocytol. 1999;28:263–70. - PubMed
-
- Bauer NG, Richter-Landsberg C, Ffrench-Constant C. Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia. 2009;57:1691–1705. - PubMed
-
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

