Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice
- PMID: 20692331
- PMCID: PMC2975513
- DOI: 10.1016/j.freeradbiomed.2010.07.026
Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice
Abstract
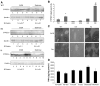
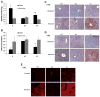
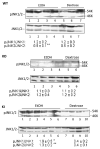
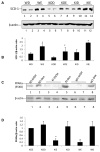
A major pathway for chronic ethanol-induced liver injury is ethanol-induced oxidant stress. Several pathways contribute to mechanisms by which ethanol induces oxidant stress. Although some studies support a role for cytochrome P450 2E1 (CYP2E1), others do not. Most previous studies were conducted in the intragastric infusion model of ethanol administration. There is a need to develop oral models of significant liver injury and to evaluate the possible role of CYP2E1 in ethanol actions in such models. We evaluated chronic ethanol-induced liver injury, steatosis, and oxidant stress in wild-type (WT) mice, CYP2E1 knock out (KO) mice, and humanized CYP2E1 knock-in (KI) mice, in which the human 2E1 was added back to mice deficient in the mouse 2E1. WT mice and the CYP2E1 KO and KI mice (both provided by Dr. F. Gonzalez, National Cancer Institute) were fed a high-fat Lieber-DeCarli ethanol liquid diet for 3weeks; pair-fed controls received dextrose. Ethanol produced fatty liver and oxidant stress in WT mice but liver injury (transaminases, histopathology) was minimal. Ethanol-induced steatosis and oxidant stress were blunted in the KO mice (no liver injury) but restored in the KI mice. Significant liver injury was produced in the ethanol-fed KI mice, with elevated transaminases, necrosis, and increased levels of collagen type 1 and smooth muscle actin. This liver injury in the KI mice was associated with elevated oxidant stress and elevated levels of the human CYP2E1 compared to levels of the mouse 2E1 in WT mice. Activation of JNK and decreased levels of Bcl-2 and Bcl-XL were observed in the ethanol-fed KI mice compared to the other groups. Fatty liver in the WT and the KI mice was associated with lower levels of PPARα and acyl-CoA oxidase. No such changes were found in the ethanol-fed KO mice. These results show that CYP2E1 plays a major role in ethanol-induced fatty liver and oxidant stress. It is the absence of CYP2E1 in the KO mice that is responsible for the blunting of steatosis and oxidant stress because restoring the CYP2E1 restores the fatty liver and oxidant stress. Moreover, it is the human CYP2E1 that restores these effects of ethanol, which suggests that results for fatty liver and oxidant stress from rodent models of ethanol intake and mouse CYP2E1 can be extrapolated to human models of ethanol intake and to human CYP2E1.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Nordman R, Riviere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Rad Biol Med. 1992;12:219–240. - PubMed
-
- Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231–242. - PubMed
-
- Cederbaum AI. Introduction serial review: alcohol, oxidative stress and cell injury. Free Rad Biol Med. 2001;31:1524–1526. - PubMed
-
- Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. - PubMed
-
- French SW, Morimoto M, Reitz RC, Koop D, Klopfenstein B, Estes, et al. Lipid peroxidation, CYP2E1 and arachidonic acid metabolism in alcoholic liver disease in rats. J Nutr. 1997;127:907S–911S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

