Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study
- PMID: 20692530
- PMCID: PMC4035014
- DOI: 10.1016/S0140-6736(10)60668-X
Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study
Abstract
Background: A common approach for tissue regeneration is cell delivery, for example by direct transplantation of stem or progenitor cells. An alternative, by recruitment of endogenous cells, needs experimental evidence. We tested the hypothesis that the articular surface of the synovial joint can regenerate with a biological cue spatially embedded in an anatomically correct bioscaffold.
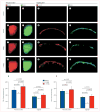
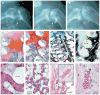
Methods: In this proof of concept study, the surface morphology of a rabbit proximal humeral joint was captured with laser scanning and reconstructed by computer-aided design. We fabricated an anatomically correct bioscaffold using a composite of poly-epsilon-caprolactone and hydroxyapatite. The entire articular surface of unilateral proximal humeral condyles of skeletally mature rabbits was surgically excised and replaced with bioscaffolds spatially infused with transforming growth factor beta3 (TGFbeta3)-adsorbed or TGFbeta3-free collagen hydrogel. Locomotion and weightbearing were assessed 1-2, 3-4, and 5-8 weeks after surgery. At 4 months, regenerated cartilage samples were retrieved from in vivo and assessed for surface fissure, thickness, density, chondrocyte numbers, collagen type II and aggrecan, and mechanical properties.
Findings: Ten rabbits received TGFbeta3-infused bioscaffolds, ten received TGFbeta3-free bioscaffolds, and three rabbits underwent humeral-head excision without bioscaffold replacement. All animals in the TGFbeta3-delivery group fully resumed weightbearing and locomotion 3-4 weeks after surgery, more consistently than those in the TGFbeta3-free group. Defect-only rabbits limped at all times. 4 months after surgery, TGFbeta3-infused bioscaffolds were fully covered with hyaline cartilage in the articular surface. TGFbeta3-free bioscaffolds had only isolated cartilage formation, and no cartilage formation occurred in defect-only rabbits. TGFbeta3 delivery yielded uniformly distributed chondrocytes in a matrix with collagen type II and aggrecan and had significantly greater thickness (p=0.044) and density (p<0.0001) than did cartilage formed without TGFbeta3. Compressive and shear properties of TGFbeta3-mediated articular cartilage did not differ from those of native articular cartilage, and were significantly greater than those of cartilage formed without TGFbeta3. Regenerated cartilage was avascular and integrated with regenerated subchondral bone that had well defined blood vessels. TGFbeta3 delivery recruited roughly 130% more cells in the regenerated articular cartilage than did spontaneous cell migration without TGFbeta3.
Interpretation: Our findings suggest that the entire articular surface of the synovial joint can regenerate without cell transplantation. Regeneration of complex tissues is probable by homing of endogenous cells, as exemplified by stratified avascular cartilage and vascularised bone. Whether cell homing acts as an adjunctive or alternative approach of cell delivery for regeneration of tissues with different organisational complexity warrants further investigation.
Funding: New York State Stem Cell Science; US National Institutes of Health.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare that we have no conflicts of interest.
Figures



Comment in
-
In-vivo tissue engineering of biological joint replacements.Lancet. 2010 Aug 7;376(9739):394-6. doi: 10.1016/S0140-6736(10)60931-2. Lancet. 2010. PMID: 20692514 No abstract available.
References
-
- Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. - PubMed
-
- Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–58. - PubMed
-
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

