Dependence on vitamin K-dependent protein S for eukaryotic cell secretion of the beta-chain of C4b-binding protein
- PMID: 20693287
- PMCID: PMC2952205
- DOI: 10.1074/jbc.M110.148452
Dependence on vitamin K-dependent protein S for eukaryotic cell secretion of the beta-chain of C4b-binding protein
Abstract
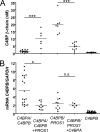
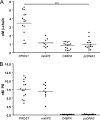
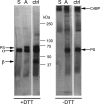
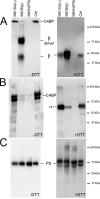
The anticoagulant vitamin K-dependent protein S (PS) circulates in plasma in two forms, 30% free and 70% being bound to the complement regulatory protein C4b-binding protein (C4BP). The major C4BP isoform consists of 7 α-chains and 1 β-chain (C4BPβ(+)), the chains being linked by disulfide bridges. PS binds to the β-chain with high affinity. In plasma, PS is in molar excess over C4BPβ(+) and due to the high affinity, all C4BPβ(+) molecules contain a bound PS. Taken together with the observation that PS-deficient patients have decreased levels of C4BPβ(+), this raises the question of whether PS is important for secretion of the β-chain from the cell. To test this hypothesis, HEK293 cells were stably and transiently transfected with β-chain cDNA in combinations with cDNAs for PS and/or the α-chain. The concentration of β-chains in the medium increased after co-transfection with PS cDNA, but not by α-chain cDNA, suggesting secretion of the β-chains from the cells to be dependent on concomitant synthesis of PS, but not of the α-chains. Thus, β-chains that were not disulfide-linked to the α-chains were secreted in complex with PS, either as monomers or dimers. Pulse-chase demonstrated that the complexes between PS and β-chain were formed intracellularly, in the endoplasmic reticulum. In conclusion, our results demonstrate that successful secretion of β-chains depends on intracellular complex formation with PS, but not on the α-chains. This provides an explanation for the decreased β-chain levels observed in PS-deficient patients.
Figures



Similar articles
-
Regulatory mechanisms of C4b-binding protein (C4BP)alpha and beta expression in rat hepatocytes by lipopolysaccharide and interleukin-6.J Thromb Haemost. 2008 Nov;6(11):1858-67. doi: 10.1111/j.1538-7836.2008.03129.x. Epub 2008 Aug 22. J Thromb Haemost. 2008. PMID: 18752574
-
High affinity binding of human vitamin K-dependent protein S to a truncated recombinant beta-chain of C4b-binding protein expressed in Escherichia coli.J Biol Chem. 1993 Feb 15;268(5):3033-6. J Biol Chem. 1993. PMID: 8428978
-
Expression and characterization of a recombinant C4b-binding protein lacking the beta-chain.Biochem J. 1995 Jun 15;308 ( Pt 3)(Pt 3):795-800. doi: 10.1042/bj3080795. Biochem J. 1995. PMID: 8948435 Free PMC article.
-
Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system.Thromb Haemost. 1991 Jul 12;66(1):49-61. Thromb Haemost. 1991. PMID: 1833851 Review.
-
Studies on the interaction between vitamin K-dependent protein S and complement regulator C4b-binding protein: localization of binding sites and identification of a possible function of the complex.Scand J Clin Lab Invest Suppl. 2002;237:19-28. doi: 10.1080/003655102762377457. Scand J Clin Lab Invest Suppl. 2002. PMID: 12570163 Review.
Cited by
-
Extracellular vesicle biomarkers for complement dysfunction in schizophrenia.Brain. 2024 Mar 1;147(3):1075-1086. doi: 10.1093/brain/awad341. Brain. 2024. PMID: 37816260 Free PMC article.
-
Serum amyloid A, protein Z, and C4b-binding protein β chain as new potential biomarkers for pulmonary tuberculosis.PLoS One. 2017 Mar 9;12(3):e0173304. doi: 10.1371/journal.pone.0173304. eCollection 2017. PLoS One. 2017. PMID: 28278182 Free PMC article.
-
Dysregulation of Protein S in COVID-19.Best Pract Res Clin Haematol. 2022 Sep;35(3):101376. doi: 10.1016/j.beha.2022.101376. Epub 2022 Aug 23. Best Pract Res Clin Haematol. 2022. PMID: 36494145 Free PMC article. Review.
-
Unfolded von Willebrand factor binds protein S and reduces anticoagulant activity.Blood Vessel Thromb Hemost. 2025 Feb;2(1):100030. doi: 10.1016/j.bvth.2024.100030. Epub 2024 Sep 25. Blood Vessel Thromb Hemost. 2025. PMID: 40519989 Free PMC article.
-
High protein S activity due to C4b-binding protein deficiency in a 34-year-old Surinamese female with ischemic retinopathy.Clin Case Rep. 2018 Mar 30;6(5):935-938. doi: 10.1002/ccr3.1464. eCollection 2018 May. Clin Case Rep. 2018. PMID: 29744091 Free PMC article.
References
-
- Dahlbäck B., Villoutreix B. O. (2005) FEBS Lett. 579, 3310–3316 - PubMed
-
- Hackeng T. M., Maurissen L. F., Castoldi E., Rosing J. (2009) J. Thromb. Haemost. 7, Suppl. 1, 165–168 - PubMed
-
- Maurissen L. F., Thomassen M. C., Nicolaes G. A., Dahlbäck B., Tans G., Rosing J., Hackeng T. M. (2008) Blood 111, 3034–3041 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

