A new role for bicarbonate in mucus formation
- PMID: 20693315
- PMCID: PMC2957415
- DOI: 10.1152/ajplung.00180.2010
A new role for bicarbonate in mucus formation
Abstract
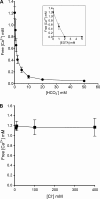
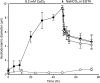
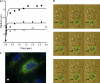
The impact of small anions on the physical properties of gel-forming mucin has been almost overlooked relative to that of cations. Recently, based on the coincident abnormalities in HCO(3)(-) secretion and abnormal mucus formed in the hereditary disease cystic fibrosis (CF), HCO(3)(-) was hypothesized to be critical in the formation of normal mucus by virtue of its ability to sequester Ca(2+) from condensed mucins being discharged from cells. However, direct evidence of the impact of HCO(3)(-) on mucus properties is lacking. Herein, we demonstrate for the first time that mucin diffusivity (∼1/viscosity) increases as a function of [HCO(3)(-)]. Direct measurements of exocytosed mucin-swelling kinetics from airway cells showed that mucin diffusivity increases by ∼300% with 20 mM extracellular HCO(3)(-) concentration. Supporting data indicate that HCO(3)(-) reduces free Ca(2+) concentration and decreases the amount of Ca(2+) that remains associated with mucins. The results demonstrate that HCO(3)(-) enhances mucin swelling and hydration by reducing Ca(2+) cross-linking in mucins, thereby decreasing its viscosity and likely increasing its transportability. In addition, HCO(3)(-) can function as a Ca(2+) chelator like EGTA to disperse mucin aggregates. This study indicates that poor HCO(3)(-) availability in CF may explain why secreted mucus remains aggregated and more viscous in affected organs. These insights bear on not only the fundamental pathogenesis in CF, but also on the process of gel mucus formation and release in general.
Figures



Similar articles
-
Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis.Lancet. 2008 Aug 2;372(9636):415-7. doi: 10.1016/S0140-6736(08)61162-9. Lancet. 2008. PMID: 18675692 Review.
-
Molecular mechanism of product storage and release in mucin secretion. II. The role of extracellular Ca++.Biorheology. 1987;24(6):625-33. doi: 10.3233/bir-1987-24615. Biorheology. 1987. PMID: 3502764
-
Mucin exocytosis.Am Rev Respir Dis. 1991 Sep;144(3 Pt 2):S33-7. doi: 10.1164/ajrccm/144.3_pt_2.S33. Am Rev Respir Dis. 1991. PMID: 1892323 Review.
-
Nanoscale Viscometry Reveals an Inherent Mucus Defect in Cystic Fibrosis.ACS Nano. 2025 Feb 4;19(4):4637-4649. doi: 10.1021/acsnano.4c14927. Epub 2025 Jan 18. ACS Nano. 2025. PMID: 39825840
-
Normal mucus formation requires cAMP-dependent HCO3- secretion and Ca2+-mediated mucin exocytosis.J Physiol. 2013 Sep 15;591(18):4581-93. doi: 10.1113/jphysiol.2013.257436. Epub 2013 Jul 1. J Physiol. 2013. PMID: 23818690 Free PMC article.
Cited by
-
The Tmem16a chloride channel is required for mucin maturation after secretion from goblet-like cells in the Xenopus tropicalis tadpole skin.Sci Rep. 2024 Oct 26;14(1):25555. doi: 10.1038/s41598-024-76482-y. Sci Rep. 2024. PMID: 39461969 Free PMC article.
-
Cystic fibrosis: an-ion transport issue?Nat Med. 2011 Feb;17(2):166-7. doi: 10.1038/nm0211-166. Nat Med. 2011. PMID: 21297610 Free PMC article.
-
The State of Weight in Cystic Fibrosis: Understanding Nutritional Status and Individualizing Nutritional Care in the Modulator Era.Nutrients. 2025 Jul 31;17(15):2533. doi: 10.3390/nu17152533. Nutrients. 2025. PMID: 40806116 Free PMC article. Review.
-
CFTR Protein: Not Just a Chloride Channel?Cells. 2021 Oct 22;10(11):2844. doi: 10.3390/cells10112844. Cells. 2021. PMID: 34831067 Free PMC article. Review.
-
Lubiprostone stimulates small intestinal mucin release.BMC Gastroenterol. 2012 Nov 6;12:156. doi: 10.1186/1471-230X-12-156. BMC Gastroenterol. 2012. PMID: 23130661 Free PMC article.
References
-
- Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol Lung Cell Mol Physiol 273: L201–L210, 1997 - PubMed
-
- Bansil R, Stanley E, LaMont JT. Mucin biophysics. Annu Rev Physiol 57: 635–657, 1995 - PubMed
-
- Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature 352: 70–73, 1991 - PubMed
-
- Baudet S, Hove-Madsen L, Bers DM. How to make and use calcium-specific mini- and microelectrodes. Methods Cell Biol 40: 93–113, 1994 - PubMed
-
- Berger JT, Voynow JA, Peters KW, Rose MC. Respiratory carcinoma cell lines. MUC genes and glycoconjugates. Am J Respir Cell Mol Biol 20: 500–510, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

