TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability
- PMID: 20693346
- PMCID: PMC2963546
- DOI: 10.2337/db09-1606
TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability
Abstract
Objective: Tumor necrosis factor-α (TNF-α) and interleukin-1 beta (IL-1β) are elevated in the vitreous of diabetic patients and in retinas of diabetic rats associated with increased retinal vascular permeability. However, the molecular mechanisms underlying retinal vascular permeability induced by these cytokines are poorly understood. In this study, the effects of IL-1β and TNF-α on retinal endothelial cell permeability were compared and the molecular mechanisms by which TNF-α increases cell permeability were elucidated.
Research design and methods: Cytokine-induced retinal vascular permeability was measured in bovine retinal endothelial cells (BRECs) and rat retinas. Western blotting, quantitative real-time PCR, and immunocytochemistry were performed to determine tight junction protein expression and localization.
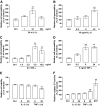
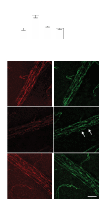
Results: IL-1β and TNF-α increased BREC permeability, and TNF-α was more potent. TNF-α decreased the protein and mRNA content of the tight junction proteins ZO-1 and claudin-5 and altered the cellular localization of these tight junction proteins. Dexamethasone prevented TNF-α-induced cell permeability through glucocorticoid receptor transactivation and nuclear factor-kappaB (NF-κB) transrepression. Preventing NF-κB activation with an inhibitor κB kinase (IKK) chemical inhibitor or adenoviral overexpression of inhibitor κB alpha (IκBα) reduced TNF-α-stimulated permeability. Finally, inhibiting protein kinase C zeta (PKCζ) using both a peptide and a novel chemical inhibitor reduced NF-κB activation and completely prevented the alterations in the tight junction complex and cell permeability induced by TNF-α in cell culture and rat retinas.
Conclusions: These results suggest that PKCζ may provide a specific therapeutic target for the prevention of vascular permeability in retinal diseases characterized by elevated TNF-α, including diabetic retinopathy.
Figures

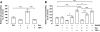




References
-
- Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IAJDRF Diabetic Retinopathy Center Group Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 2006;55:2401–2411 - PubMed
-
- Gardner TW, Larsen M, Girach A, Zhi Xon behalf of the Protein Kinase C Diabetic Retinopathy Study (PCK-DRS2) Study Group Diabetic macular oedema and visual loss: relationship to location, severity and duration. Acta Ophthalmol 2009;87:709–713 - PubMed
-
- Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology 1998;105:998–1003 - PubMed
-
- Sander B, Thornit DN, Colmorn L, Strøm C, Girach A, Hubbard LD, Lund-Andersen H, Larsen M. Progression of diabetic macular edema: correlation with blood retinal barrier permeability, retinal thickness, and retinal vessel diameter. Invest Ophthalmol Vis Sci 2007;48:3983–3987 - PubMed
-
- Diabetic Retinopathy Clinical Research Network, Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, Bressler NM, Danis RP, Kinyoun JL, Nguyen QD, Bhavsar AR, Gottlieb J, Pieramici DJ, Rauser ME, Apte RS, Lim JI, Miskala PH. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007;114:525–536 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

