AMP-activated protein kinase α2 subunit is required for the preservation of hepatic insulin sensitivity by n-3 polyunsaturated fatty acids
- PMID: 20693347
- PMCID: PMC2963531
- DOI: 10.2337/db09-1716
AMP-activated protein kinase α2 subunit is required for the preservation of hepatic insulin sensitivity by n-3 polyunsaturated fatty acids
Abstract
Objective: The induction of obesity, dyslipidemia, and insulin resistance by high-fat diet in rodents can be prevented by n-3 long-chain polyunsaturated fatty acids (LC-PUFAs). We tested a hypothesis whether AMP-activated protein kinase (AMPK) has a role in the beneficial effects of n-3 LC-PUFAs.
Research design and methods: Mice with a whole-body deletion of the α2 catalytic subunit of AMPK (AMPKα2(-/-)) and their wild-type littermates were fed on either a low-fat chow, or a corn oil-based high-fat diet (cHF), or a cHF diet with 15% lipids replaced by n-3 LC-PUFA concentrate (cHF+F).
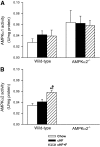
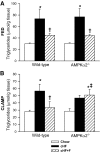
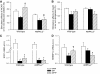
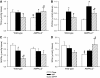
Results: Feeding a cHF diet induced obesity, dyslipidemia, hepatic steatosis, and whole-body insulin resistance in mice of both genotypes. Although cHF+F feeding increased hepatic AMPKα2 activity, the body weight gain, dyslipidemia, and the accumulation of hepatic triglycerides were prevented by the cHF+F diet to a similar degree in both AMPKα2(-/-) and wild-type mice in ad libitum-fed state. However, preservation of hepatic insulin sensitivity by n-3 LC-PUFAs required functional AMPKα2 and correlated with the induction of adiponectin and reduction in liver diacylglycerol content. Under hyperinsulinemic-euglycemic conditions, AMPKα2 was essential for preserving low levels of both hepatic and plasma triglycerides, as well as plasma free fatty acids, in response to the n-3 LC-PUFA treatment.
Conclusions: Our results show that n-3 LC-PUFAs prevent hepatic insulin resistance in an AMPKα2-dependent manner and support the role of adiponectin and hepatic diacylglycerols in the regulation of insulin sensitivity. AMPKα2 is also essential for hypolipidemic and antisteatotic effects of n-3 LC-PUFA under insulin-stimulated conditions.
Figures
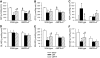
References
-
- Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci 2009;116:1–16 - PubMed
-
- Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc 2005;105:428–440 - PubMed
-
- Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ. Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr 1999;70:817–825 - PubMed
-
- Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes 1997;21:637–643 - PubMed
-
- Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Veck M, Tvrzicka E, Bryhn M, Kopecky J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004;39:1177–1185 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

