ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize
- PMID: 20693409
- PMCID: PMC2955750
- DOI: 10.1093/jxb/erq243
ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize
Abstract
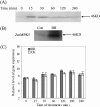
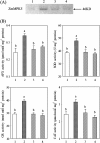
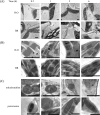
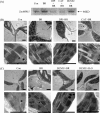
Brassinosteroids (BRs) have been shown to induce hydrogen peroxide (H(2)O(2)) accumulation, and BR-induced H(2)O(2) up-regulates antioxidant defence systems in plants. However, the mechanisms by which BR-induced H(2)O(2) regulates antioxidant defence systems in plants remain to be determined. In the present study, the role of ZmMPK5, a mitogen-activated protein kinase, in BR-induced anitioxidant defence and the relationship between the activation of ZmMPK5 and H(2)O(2) production in BR signalling were investigated in leaves of maize (Zea mays) plants. BR treatment activated ZmMPK5, induced apoplastic and chloroplastic H(2)O(2) accumulation, and enhanced the total activities of antioxidant enzymes. Such enhancements were blocked by pre-treatment with mitogen-activated protein kinase kinase (MAPKK) inhibitors and H(2)O(2) inhibitors or scavengers. Pre-treatment with MAPKK inhibitors substantially arrested the BR-induced apoplastic H(2)O(2) production after 6 h of BR treatment, but did not affect the levels of apoplastic H(2)O(2) within 1 h of BR treatment. BR-induced gene expression of NADPH oxidase was also blocked by pre-treatment with MAPKK inhibitors and an apoplastic H(2)O(2) inhibitor or scavenger after 120 min of BR treatment, but was not affected within 30 min of BR treatment. These results suggest that the BR-induced initial apoplastic H(2)O(2) production activates ZmMPK5, which is involved in self-propagation of apoplastic H(2)O(2) via regulation of NADPH oxidase gene expression in BR-induced antioxidant defence systems.
Figures




Similar articles
-
MAP65-1a positively regulates H2O2 amplification and enhances brassinosteroid-induced antioxidant defence in maize.J Exp Bot. 2013 Sep;64(12):3787-802. doi: 10.1093/jxb/ert215. J Exp Bot. 2013. PMID: 23956414 Free PMC article.
-
Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling.J Exp Bot. 2009;60(11):3221-38. doi: 10.1093/jxb/erp157. Epub 2009 Jul 10. J Exp Bot. 2009. PMID: 19592501 Free PMC article.
-
Calcium and ZmCCaMK are involved in brassinosteroid-induced antioxidant defense in maize leaves.Plant Cell Physiol. 2015 May;56(5):883-96. doi: 10.1093/pcp/pcv014. Epub 2015 Feb 2. Plant Cell Physiol. 2015. PMID: 25647327
-
Brassinosteroid-promoted growth.Plant Biol (Stuttg). 2005 Mar;7(2):110-7. doi: 10.1055/s-2005-837493. Plant Biol (Stuttg). 2005. PMID: 15822006 Review.
-
Brassinosteroids signal through two receptor-like kinases.Curr Opin Plant Biol. 2003 Oct;6(5):494-9. doi: 10.1016/s1369-5266(03)00088-8. Curr Opin Plant Biol. 2003. PMID: 12972051 Review.
Cited by
-
Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds.J Exp Bot. 2012 Mar;63(5):1809-22. doi: 10.1093/jxb/err336. Epub 2011 Dec 26. J Exp Bot. 2012. PMID: 22200664 Free PMC article.
-
Overexpression of a Zea mays Brassinosteroid-Signaling Kinase Gene ZmBSK1 Confers Salt Stress Tolerance in Maize.Front Plant Sci. 2022 May 6;13:894710. doi: 10.3389/fpls.2022.894710. eCollection 2022. Front Plant Sci. 2022. PMID: 35599886 Free PMC article.
-
Comparative Histological and Transcriptional Analysis of Maize Kernels Infected with Aspergillus flavus and Fusarium verticillioides.Front Plant Sci. 2017 Dec 6;8:2075. doi: 10.3389/fpls.2017.02075. eCollection 2017. Front Plant Sci. 2017. PMID: 29270183 Free PMC article.
-
Identification of the metallothionein gene family from cucumber and functional characterization of CsMT4 in Escherichia coli under salinity and osmotic stress.3 Biotech. 2019 Nov;9(11):394. doi: 10.1007/s13205-019-1929-8. Epub 2019 Oct 11. 3 Biotech. 2019. PMID: 31656732 Free PMC article.
-
RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato.J Exp Bot. 2014 Feb;65(2):595-607. doi: 10.1093/jxb/ert404. Epub 2013 Dec 9. J Exp Bot. 2014. PMID: 24323505 Free PMC article.
References
-
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. - PubMed
-
- Bajguz A, Hayat S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology and Biochemistry. 2009;47:1–8. - PubMed
-
- Bartoli CG, Gómez F, Martínez DE, Guiamet JJ. Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.) Journal of Experimental Botany. 2004;55:1663–1669. - PubMed
-
- Berberich T, Sano H, Kusano T. Involvement of a MAP kinase, ZmMPK5, in senescence and recovery from low-temperature stress in maize. Molecular and General Genetics. 1999;262:534–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

