PPARγ activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease
- PMID: 20693431
- PMCID: PMC2996113
- DOI: 10.1182/blood-2010-02-272005
PPARγ activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease
Abstract
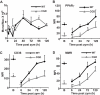
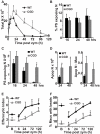
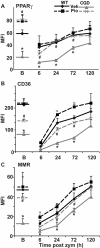
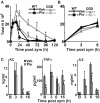
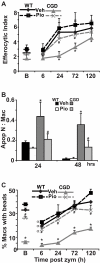
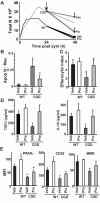
Absence of a functional nicotinamide adenine dinucleotide phosphate (NADPH) oxidase predisposes chronic granulomatous disease (CGD) patients to infection, and also to unexplained, exaggerated inflammation. The impaired recognition and removal (efferocytosis) of apoptotic neutrophils by CGD macrophages may contribute to this effect. We hypothesized that peroxisome proliferator-activated receptor γ (PPARγ) activation during CGD inflammation is deficient, leading to altered macrophage programming and decreased efferocytosis, and that PPARγ agonism would enhance resolution. using the gp91(phox-/-) murine model of X-linked CGD in a well-characterized model of sterile, zymosan-induced peritonitis, it was demonstrated that PPARγ expression and activation in CGD macrophages were significantly deficient at baseline, and acquisition was delayed over the course of inflammation relative to that of wild-type. Efferocytosis by macrophages reflected PPARγ activation during peritonitis and was impaired in CGD mice (versus wild-type), leading to accumulation of apoptotic neutrophils. Importantly, provision of the PPARγ agonist, pioglitazone, either prophylactically or during inflammation, significantly enhanced macrophage PPARγ-mediated programming and efferocytosis, reduced accumulation of apoptotic neutrophils, and normalized the course of peritonitis in CGD mice. As such, PPARγ may be a therapeutic target for CGD, and possibly other inflammatory conditions where aberrant macrophage programming and impaired efferocytosis delay resolution of inflammation.
Figures

References
-
- Johnston RB., Jr Clinical aspects of chronic granulomatous disease. Curr Opin Hematol. 2001;8(1):17–22. - PubMed
-
- Rupec RA, Petropoulou T, Belohradsky BH, et al. Lupus erythematosus tumidus and chronic discoid lupus erythematosus in carriers of X-linked chronic granulomatous disease. Eur J Dermatol. 2000;10(3):184–189. - PubMed
-
- Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79(3):170–200. - PubMed
-
- Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38(1):3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

