Design of thermostable beta-propeller phytases with activity over a broad range of pHs and their overproduction by Pichia pastoris
- PMID: 20693453
- PMCID: PMC2950461
- DOI: 10.1128/AEM.00253-10
Design of thermostable beta-propeller phytases with activity over a broad range of pHs and their overproduction by Pichia pastoris
Abstract
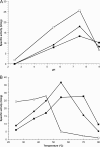
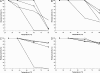
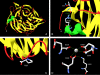
Thermostable phytases, which are active over broad pH ranges, may be useful as feed additives, since they can resist the temperatures used in the feed-pelleting process. We designed new beta-propeller phytases, using a structure-guided consensus approach, from a set of amino acid sequences from Bacillus phytases and engineered Pichia pastoris strains to overproduce the enzymes. The recombinant phytases were N-glycosylated, had the correct amino-terminal sequence, showed activity over a pH range of 2.5 to 9, showed a high residual activity after 10 min of heat treatment at 80°C and pH 5.5 or 7.5, and were more thermostable at pH 7.5 than a recombinant form of phytase C from Bacillus subtilis (GenBank accession no. AAC31775). A structural analysis suggested that the higher thermostability may be due to a larger number of hydrogen bonds and to the presence of P257 in a surface loop. In addition, D336 likely plays an important role in the thermostability of the phytases at pH 7.5. The recombinant phytases showed higher thermostability at pH 5.5 than at pH 7.5. This difference was likely due to a different protein total charge at pH 5.5 from that at pH 7.5. The recombinant beta-propeller phytases described here may have potential as feed additives and in the pretreatment of vegetable flours used as ingredients in animal diets.
Figures

Similar articles
-
Expression of a Bacillus phytase C gene in Pichia pastoris and properties of the recombinant enzyme.Appl Environ Microbiol. 2010 Aug;76(16):5601-8. doi: 10.1128/AEM.00762-10. Epub 2010 Jul 2. Appl Environ Microbiol. 2010. PMID: 20601512 Free PMC article.
-
Overexpression and biochemical characterization of a thermostable phytase from Bacillus subtilis US417 in Pichia pastoris.Mol Biotechnol. 2014 Sep;56(9):839-48. doi: 10.1007/s12033-014-9764-y. Mol Biotechnol. 2014. PMID: 24859267
-
Expression and characterization of Aspergillus thermostable phytases in Pichia pastoris.FEMS Microbiol Lett. 2009 Jan;290(1):18-24. doi: 10.1111/j.1574-6968.2008.01399.x. Epub 2008 Nov 12. FEMS Microbiol Lett. 2009. PMID: 19025560
-
Molecular advancements in the development of thermostable phytases.Appl Microbiol Biotechnol. 2017 Apr;101(7):2677-2689. doi: 10.1007/s00253-017-8195-7. Epub 2017 Feb 23. Appl Microbiol Biotechnol. 2017. PMID: 28233043 Review.
-
Bacillus phytases: Current status and future prospects.Bioengineered. 2015;6(4):233-6. doi: 10.1080/21655979.2015.1048050. Epub 2015 May 6. Bioengineered. 2015. PMID: 25946551 Free PMC article. Review.
Cited by
-
Improved thermostability of Clostridium thermocellum endoglucanase Cel8A by using consensus-guided mutagenesis.Appl Environ Microbiol. 2012 May;78(9):3458-64. doi: 10.1128/AEM.07985-11. Epub 2012 Mar 2. Appl Environ Microbiol. 2012. PMID: 22389377 Free PMC article.
-
Consensus protein engineering on the thermostable histone-like bacterial protein HUs significantly improves stability and DNA binding affinity.Extremophiles. 2020 Mar;24(2):293-306. doi: 10.1007/s00792-020-01154-4. Epub 2020 Jan 24. Extremophiles. 2020. PMID: 31980943
-
Development and applications of artificial symmetrical proteins.Comput Struct Biotechnol J. 2020 Nov 27;18:3959-3968. doi: 10.1016/j.csbj.2020.10.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33335692 Free PMC article. Review.
-
In silico and in vitro studies of cytotoxic activity of different peptides derived from vesicular stomatitis virus G protein.Iran J Basic Med Sci. 2015 Jan;18(1):47-52. Iran J Basic Med Sci. 2015. PMID: 25810875 Free PMC article.
-
Effect of AOX1 and GAP transcriptional terminators on transcript levels of both the heterologous and the GAPDH genes and the extracellular Yp/x in GAP promoter-based Komagataella phaffii strains.PeerJ. 2024 Sep 26;12:e18181. doi: 10.7717/peerj.18181. eCollection 2024. PeerJ. 2024. PMID: 40109886 Free PMC article.
References
-
- Barzegar, A., A. A. Moosavi-Movahedi, J. Z. Pedersen, and M. Miroliaei. 2009. Comparative thermostability of mesophilic and thermophilic alcohol dehydrogenases: stability-determining roles of proline residues and loop conformations. Enzyme Microb. Technol. 45:73-79.
-
- Byrne, L. J., K. J. O'Callaghan, and M. F. Tuite. 2005. Heterologous gene expression in yeast. Methods Mol. Biol. 308:51-64. - PubMed
-
- Choi, E. J., and S. L. Mayo. 2006. Generation and analysis of proline mutants in protein G. Protein Eng. Des. Sel. 19:285-289. - PubMed
-
- Clark, S. E., E. H. Muslin, and C. H. Henson. 2004. Effect of adding and removing N-glycosylation recognition sites on the thermostability of barley α-glucosidase. Protein Eng. Des. Sel. 17:245-249. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

