Dendritic cells: are they clinically relevant?
- PMID: 20693842
- PMCID: PMC2919819
- DOI: 10.1097/PPO.0b013e3181eaca83
Dendritic cells: are they clinically relevant?
Abstract
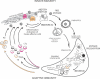
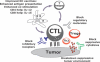
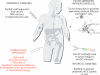
Cancer vaccines have undergone a renaissance because of recent clinical trials showing promising immunologic data and some clinical benefit to patients. Current trials exploiting dendritic cells (DCs) as vaccines have shown durable tumor regressions in a fraction of patients. Clinical efficacy of current vaccines is hampered by myeloid-derived suppressor cells, inflammatory type 2 T cells, and regulatory T cells, all of which prevent the generation of effector cells. To improve the clinical efficacy of DC vaccines, we need to design novel and improved strategies that can boost adaptive immunity to cancer, help overcome regulatory T cells and allow the breakdown of the immunosuppressive tumor microenvironment. This can be achieved by exploiting the fast increasing knowledge about the DC system, including the existence of distinct DC subsets. Critical to the design of better vaccines is the concept of distinct DC subsets and distinct DC activation pathways, all contributing to the generation of unique adaptive immune responses. Such novel DC vaccines will be used as monotherapy in patients with resected disease and in combination with antibodies and/or drugs targeting suppressor pathways and modulation of the tumor environment in patients with metastatic disease.
Figures

References
-
- Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. - PubMed
-
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002 - PMC - PubMed
-
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. - PMC - PubMed
-
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

