Adoptive cell therapy: genetic modification to redirect effector cell specificity
- PMID: 20693844
- PMCID: PMC6348476
- DOI: 10.1097/PPO.0b013e3181eb3879
Adoptive cell therapy: genetic modification to redirect effector cell specificity
Abstract
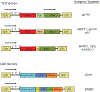
Building on the principals that the adoptive transfer of T cells can lead to the regression of established tumors in humans, investigators are now further manipulating these cells using genetic engineering. Two decades of human gene transfer experiments have resulted in the translation of laboratory technology into robust clinical applications. The purpose of this review is to give the reader an introduction to the 2 major approaches being developed to redirect effector T-cell specificity. Primary human T cells can be engineered to express exogenous T-cell receptors or chimeric antigen receptors directed against multiple human tumor antigens. Initial clinical trial results have demonstrated that both T-cell receptor- and chimeric antigen receptor-engineered T cells can be administered to cancer patients and mediate tumor regression.
Figures

References
-
- Dembic Z, Haas W, Weiss S, et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986;320:232–238. - PubMed
-
- Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990; 323:570–578. - PubMed
-
- Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. - PubMed
-
- Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

