Indoleamine 2,3-dioxygenase: is it an immune suppressor?
- PMID: 20693847
- PMCID: PMC3850167
- DOI: 10.1097/PPO.0b013e3181eb3343
Indoleamine 2,3-dioxygenase: is it an immune suppressor?
Abstract
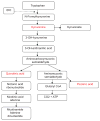
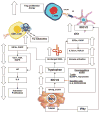
This article covers what is currently known about the role of the enzyme indoleamine 2,3-dioxygenase (IDO) in cancer-related immunosuppression and the clinical research on IDO inhibitors. A PUBMED search was performed using the terms IDO, indoleamine 2,3-dioxygenase, 1-MT. IDO is an inducible enzyme that catalyzes the rate-limiting first step in tryptophan catabolism. This enzyme is overexpressed in response to IFNgamma in a variety of different malignancies. IDO causes immunosuppression through breakdown of tryptophan in the tumor microenvironment and tumor-draining lymph nodes. The depletion of tryptophan and toxic catabolites renders effector T cells inactive and dendritic cells immunosuppressive. Preclinical data suggest that IDO inhibition can delay tumor growth, enhance dendritic cell vaccines, and synergize with chemotherapy through immune-mediated mechanisms. The lead IDO inhibitor, d-1-methyl-tryptophan (d-1-MT), was selected for phase I trials and seems to have immune modulating activity. Subsequently, another isoform of IDO, IDO2, was discovered and found to be the target of d-1-MT. Multiple single-nucleotide polymorphisms in IDO2 affecting its catalytic activity may serve as a pharmacogenetic predictive biomarker for d-1-MT. The IDO pathway is an important mechanism of tumor-related immunosuppression and blocking it could improve cancer immunotherapy outcomes. Clinical development of d-1-MT and other IDO inhibitors as systemic immunomodulators to be combined with other immune modulators, vaccines, and chemotherapy are ongoing.
Figures
Similar articles
-
Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape.Immunol Rev. 2008 Apr;222:206-21. doi: 10.1111/j.1600-065X.2008.00610.x. Immunol Rev. 2008. PMID: 18364004 Review.
-
Indoleamine 2,3-dioxygenase (IDO): Biology and Target in Cancer Immunotherapies.Curr Cancer Drug Targets. 2016;16(9):755-764. doi: 10.2174/1568009615666151030102250. Curr Cancer Drug Targets. 2016. PMID: 26517538 Review.
-
Indoleamine 2,3-dioxygenase in immune suppression and cancer.Curr Cancer Drug Targets. 2007 Feb;7(1):31-40. doi: 10.2174/156800907780006896. Curr Cancer Drug Targets. 2007. PMID: 17305476 Review.
-
Influence of tryptophan contained in 1-Methyl-Tryptophan on antimicrobial and immunoregulatory functions of indoleamine 2,3-dioxygenase.PLoS One. 2012;7(9):e44797. doi: 10.1371/journal.pone.0044797. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028625 Free PMC article.
-
Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan.Cancer Res. 2007 Aug 1;67(15):7082-7. doi: 10.1158/0008-5472.CAN-07-1872. Cancer Res. 2007. PMID: 17671174
Cited by
-
Adoptive Cell Transfer: Is it a Promising Immunotherapy for Colorectal Cancer?Theranostics. 2018 Nov 10;8(20):5784-5800. doi: 10.7150/thno.29035. eCollection 2018. Theranostics. 2018. PMID: 30555581 Free PMC article. Review.
-
Hypoxia and the Kynurenine Pathway: Implications and Therapeutic Prospects in Alzheimer's Disease.Oxid Med Cell Longev. 2021 Nov 10;2021:5522981. doi: 10.1155/2021/5522981. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34804368 Free PMC article. Review.
-
Tryptophan Catabolism in Chronic Viral Infections: Handling Uninvited Guests.Int J Tryptophan Res. 2015 Aug 4;8:41-8. doi: 10.4137/IJTR.S26862. eCollection 2015. Int J Tryptophan Res. 2015. PMID: 26309411 Free PMC article. Review.
-
Necroptosis in neurodegenerative diseases: a potential therapeutic target.Cell Death Dis. 2017 Jun 29;8(6):e2905. doi: 10.1038/cddis.2017.286. Cell Death Dis. 2017. PMID: 28661482 Free PMC article. Review.
-
Redox control in the pathophysiology of influenza virus infection.BMC Microbiol. 2020 Jul 20;20(1):214. doi: 10.1186/s12866-020-01890-9. BMC Microbiol. 2020. PMID: 32689931 Free PMC article. Review.
References
-
- Bickels J, Kollender Y, Merinsky O, et al. Coley’s toxin: historical perspective. Isr Med Assoc J. 2002;4:471– 472. - PubMed
-
- Hoption Cann SA, van Netten JP, van Netten C, et al. Spontaneous regression: a hidden treasure buried in time. Med Hypotheses. 2002;58:115–119. - PubMed
-
- Higuchi K, Hayaishi O. Enzymic formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys. 1967;120:397– 403. - PubMed
-
- Yamamoto S, Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967;242:5260–5266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

