PTEN deletion enhances the regenerative ability of adult corticospinal neurons
- PMID: 20694004
- PMCID: PMC2928871
- DOI: 10.1038/nn.2603
PTEN deletion enhances the regenerative ability of adult corticospinal neurons
Abstract
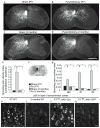
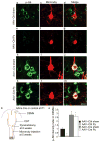
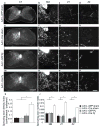
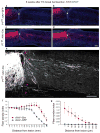
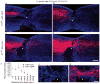
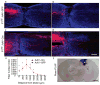
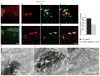
Despite the essential role of the corticospinal tract (CST) in controlling voluntary movements, successful regeneration of large numbers of injured CST axons beyond a spinal cord lesion has never been achieved. We found that PTEN/mTOR are critical for controlling the regenerative capacity of mouse corticospinal neurons. After development, the regrowth potential of CST axons was lost and this was accompanied by a downregulation of mTOR activity in corticospinal neurons. Axonal injury further diminished neuronal mTOR activity in these neurons. Forced upregulation of mTOR activity in corticospinal neurons by conditional deletion of Pten, a negative regulator of mTOR, enhanced compensatory sprouting of uninjured CST axons and enabled successful regeneration of a cohort of injured CST axons past a spinal cord lesion. Furthermore, these regenerating CST axons possessed the ability to reform synapses in spinal segments distal to the injury. Thus, modulating neuronal intrinsic PTEN/mTOR activity represents a potential therapeutic strategy for promoting axon regeneration and functional repair after adult spinal cord injury.
Figures
References
-
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. - PubMed
-
- Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. - PubMed
-
- Zheng B, Lee JK, Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends Neurosci. 2006;29:640–646. - PubMed
-
- Deumens R, Koopmans GC, Joosten EA. Regeneration of descending axon tracts after spinal cord injury. Prog Neurobiol. 2005;77:57–89. - PubMed
-
- Thallmair M, Metz GA, Z’Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1:124–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

