Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia
- PMID: 20694012
- PMCID: PMC4297285
- DOI: 10.1038/ng.641
Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia
Abstract
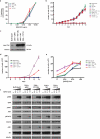
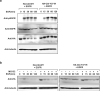
CBL encodes a member of the Cbl family of proteins, which functions as an E3 ubiquitin ligase. We describe a dominant developmental disorder resulting from germline missense CBL mutations, which is characterized by impaired growth, developmental delay, cryptorchidism and a predisposition to juvenile myelomonocytic leukemia (JMML). Some individuals experienced spontaneous regression of their JMML but developed vasculitis later in life. Importantly, JMML specimens from affected children show loss of the normal CBL allele through acquired isodisomy. Consistent with these genetic data, the common p.371Y>H altered Cbl protein induces cytokine-independent growth and constitutive phosphorylation of ERK, AKT and S6 only in hematopoietic cells in which normal Cbl expression is reduced by RNA interference. We conclude that germline CBL mutations have developmental, tumorigenic and functional consequences that resemble disorders that are caused by hyperactive Ras/Raf/MEK/ERK signaling and include neurofibromatosis type 1, Noonan syndrome, Costello syndrome, cardiofaciocutaneous syndrome and Legius syndrome.
Figures


References
-
- Niemeyer CM, Kratz CP. Paediatric myelodysplastic syndromes and juvenile myelomonocytic leukaemia: molecular classification and treatment options. Br J Haematol. 2008;140:610–24. - PubMed
-
- Locatelli F, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105:410–9. - PubMed
-
- Matsuda K, et al. Spontaneous improvement of hematologic abnormalities in patients having juvenile myelomonocytic leukemia with specific RAS mutations. Blood. 2007;109:5477–80. - PubMed
-
- Flotho C, et al. Genotype-phenotype correlation in cases of juvenile myelomonocytic leukemia with clonal RAS mutations. Blood. 2008;111:966–7. author reply 967-8. - PubMed
-
- Niemeyer CM, et al. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS). Blood. 1997;89:3534–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

