Raltegravir: The evidence of its therapeutic value in HIV-1 infection
- PMID: 20694070
- PMCID: PMC2899791
- DOI: 10.2147/ce.s6004
Raltegravir: The evidence of its therapeutic value in HIV-1 infection
Abstract
Introduction: The antiretroviral treatment paradigm for human immunodeficiency virus-1 (HIV-1) infection has undergone a significant change with the addition of a new class of therapeutic agents targeting HIV-1 integrase (IN). IN inhibitors prevent the integration of viral DNA into the human genome and terminate the viral life cycle. As the first member of this new class of anti-HIV drugs, raltegravir has shown promising results in the clinic.
Aims: To review the emerging evidence for the use of the IN inhibitor raltegravir in the treatment of HIV-1 infection.
Evidence review: Strong evidence shows that raltegravir is effective in reducing the viral load to less than 50 copies/mL and increasing CD4 cell count in treatment-experienced patients with triple-drug class-resistant HIV-1 infection. Substantial evidence also indicates that while raltegravir is able to achieve treatment response in patients with drug-resistant HIV-1, it is susceptible to development of resistance. Raltegravir should be used with at least one other active drug. In addition to its use in salvage therapy upon failure of first-line antiretroviral treatment, a raltegravir-based treatment regimen may also be effective as initial therapy. Substantial evidence also shows that raltegravir-based treatment regimen is well tolerated with minimal clinically severe adverse events and toxicities. Modeling studies suggest a cost-effectiveness of US$21,339 per quality-adjusted life year gained with raltegravir use, though further direct evidence on quality of life and cost-effectiveness is needed.
Place in therapy: Raltegravir shows significant and sustained virologic and immunologic response in combination with other antiretrovirals in treatment-experienced HIV-1 infected patients who show evidence of viral replication or multidrug-resistant HIV-1 strains, without any significant tolerability issues.
Keywords: HIV-1; MK-0518; clinical evidence; integrase inhibitor; isentress; raltegravir.
Figures

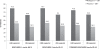

References
-
- UNAIDS Report on the global HIV/AIDS epidemic 2008: executive summary 2008. Accessed February 2008. Available from: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_...
-
- Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. - PubMed
-
- Emmelkamp JM, Rockstroh JK. Maraviroc, risks and benefits: a review of the clinical literature. Expert Opin Drug Safety. 2008;7:559–569. - PubMed
-
- Montaner J, Yeni P, Clumeck NN, et al. Safety, tolerability, and preliminary efficacy of 48 weeks of etravirine therapy in a phase IIb dose-ranging study involving treatment-experienced patients with HIV-1 infection. Clin Infect Dis. 2008;47:969–978. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

