Copper-dependent trafficking of the Ctr4-Ctr5 copper transporting complex
- PMID: 20694150
- PMCID: PMC2915924
- DOI: 10.1371/journal.pone.0011964
Copper-dependent trafficking of the Ctr4-Ctr5 copper transporting complex
Abstract
Background: In Schizosaccharomyces pombe, copper uptake is carried out by a heteromeric complex formed by the Ctr4 and Ctr5 proteins. Copper-induced differential subcellular localization may play a critical role with respect to fine tuning the number of Ctr4 and Ctr5 molecules at the cell surface.
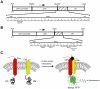
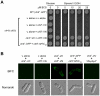
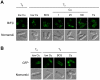
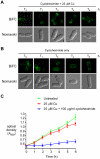
Methodology/principal findings: We have developed a bimolecular fluorescence complementation (BiFC) assay to analyze protein-protein interactions in vivo in S. pombe. The assay is based on the observation that N- and C-terminal subfragments of the Venus fluorescent protein can reconstitute a functional fluorophore only when they are brought into tight contact. Wild-type copies of the ctr4(+) and ctr5(+) genes were inserted downstream of and in-frame with the nonfluorescent C-terminal (VC) and N-terminal (VN) coding fragments of Venus, respectively. Co-expression of Ctr4-VC and Ctr5-VN fusion proteins allowed their detection at the plasma membrane of copper-limited cells. Similarly, cells co-expressing Ctr4-VN and Ctr4-VC in the presence of Ctr5-Myc(12) displayed a fluorescence signal at the plasma membrane. In contrast, Ctr5-VN and Ctr5-VC co-expressed in the presence of Ctr4-Flag(2) failed to be visualized at the plasma membrane, suggesting a requirement for a combination of two Ctr4 molecules with one Ctr5 molecule. We found that plasma membrane-located Ctr4-VC-Ctr5-VN fluorescent complexes were internalized when the cells were exposed to high levels of copper. The copper-induced internalization of Ctr4-VC-Ctr5-VN complexes was not dependent on de novo protein synthesis. When cells were transferred back from high to low copper levels, there was reappearance of the BiFC fluorescent signal at the plasma membrane.
Significance: These findings reveal a copper-dependent internalization and recycling of the heteromeric Ctr4-Ctr5 complex as a function of copper availability.
Conflict of interest statement
Figures


References
-
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. - PubMed
-
- Halliwell B, Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992;307:108–112. - PubMed
-
- Puig S, Thiele DJ. Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol. 2002;6:171–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

