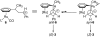Chain- and Regioselective Ethylene and Styrene Dimerization Reactions Catalyzed by a Well-Defined Cationic Ruthenium-Hydride Complex: New Insights on the Styrene Dimerization Mechanism
- PMID: 20694188
- PMCID: PMC2915581
- DOI: 10.1021/om100468q
Chain- and Regioselective Ethylene and Styrene Dimerization Reactions Catalyzed by a Well-Defined Cationic Ruthenium-Hydride Complex: New Insights on the Styrene Dimerization Mechanism
Abstract
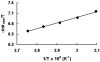
The cationic ruthenium-hydride complex [(eta(6)-C(6)H(6))(PCy(3))(CO)RuH](+)BF(4) (-) was found to be a highly regioselective catalyst for the ethylene dimerization reaction to give 2-butene products (TOF = 1910 h(-1), >95% selectivity for 2-butenes). The dimerization of styrene exclusively produced the head-to-tail dimer (E)-PhCH(CH(3))CH=CHPh at an initial turnover rate of 2300 h(-1). A rapid and extensive H/D exchange between the vinyl hydrogens of styrene-d(8) and 4-methoxystyrene was observed within 10 min without forming the dimer products at room temperature. The inverse deuterium isotope effect of k(H)/k(D) = 0.77+/-0.10 was measured from the first order plots on the dimerization reaction of styrene and styrene-d(8) in chlorobenzene at 70 degrees C. The pronounced carbon isotope effect on both vinyl carbons of styrene as measured by using Singleton's method ((13)C(recovered)/(13)C(virgin) at C(1) = 1.096 and C(2) = 1.042) indicates that the C-C bond formation is the rate-limiting step for the dimerization reaction. The Eyring plot of the dimerization of styrene in the temperature range of 50-90 degrees C led to DeltaH(double dagger) = 3.3(6) kcal/mol and DeltaS(double dagger) = -35.5(7) e.u. An electrophilic addition mechanism has been proposed for the dimerization of styrene.
Figures
Similar articles
-
Chelate-Assisted Oxidative Coupling Reaction of Arylamides and Unactivated Alkenes: Mechanistic Evidence for Vinyl C-H Bond Activation Promoted by an Electrophilic Ruthenium-Hydride Catalyst.Organometallics. 2010 Oct 21;29(22):5748-5750. doi: 10.1021/om100764c. Organometallics. 2010. PMID: 21344062 Free PMC article.
-
Scope and Mechanistic Study of the Coupling Reaction of α, β-Unsaturated Carbonyl Compounds with Alkenes: Uncovering Electronic Effects on Alkene Insertion vs Oxidative Coupling Pathways.Organometallics. 2012 Jan 9;31(1):495-504. doi: 10.1021/om201190v. Organometallics. 2012. PMID: 22368318 Free PMC article.
-
Kinetics and thermodynamics of H. transfer from (eta5-C5R5)Cr(CO)3H (R = Ph, Me, H) to methyl methacrylate and styrene.J Am Chem Soc. 2003 Aug 20;125(33):10093-102. doi: 10.1021/ja034927l. J Am Chem Soc. 2003. PMID: 12914473
-
Scope and mechanistic study of the ruthenium-catalyzed ortho-C-H bond activation and cyclization reactions of arylamines with terminal alkynes.J Am Chem Soc. 2005 Dec 7;127(48):17000-6. doi: 10.1021/ja055608s. J Am Chem Soc. 2005. PMID: 16316246 Free PMC article.
-
Structure and reactivity of bis(silyl) dihydride complexes (PMe(3))(3)Ru(SiR(3))(2)(H)(2): model compounds and real intermediates in a dehydrogenative C-Si bond forming reaction.J Am Chem Soc. 2003 Jul 23;125(29):8936-48. doi: 10.1021/ja035916v. J Am Chem Soc. 2003. PMID: 12862491
Cited by
-
Intermolecular Markovnikov-Selective Hydroacylation of Olefins Catalyzed by a Cationic Ruthenium-Hydride Complex.ACS Catal. 2016 May 6;6(5):3336-3339. doi: 10.1021/acscatal.6b00856. Epub 2016 Apr 19. ACS Catal. 2016. PMID: 30505623 Free PMC article.
-
Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization.Molecules. 2020 May 8;25(9):2216. doi: 10.3390/molecules25092216. Molecules. 2020. PMID: 32397335 Free PMC article.
-
Experimental and Computational Study of the ( Z)-Selective Formation of Trisubstituted Olefins and Benzo-Fused Oxacycles from the Ruthenium-Catalyzed Dehydrative C-H Coupling of Phenols with Ketones.J Am Chem Soc. 2018 Aug 15;140(32):10289-10296. doi: 10.1021/jacs.8b05875. Epub 2018 Aug 3. J Am Chem Soc. 2018. PMID: 30032611 Free PMC article.
-
New chiral P-N ligands for the regio- and stereoselective Pd-catalyzed dimerization of styrene.Molecules. 2011 Feb 22;16(2):1804-24. doi: 10.3390/molecules16021804. Molecules. 2011. PMID: 21343886 Free PMC article.
-
Chelate-Assisted Oxidative Coupling Reaction of Arylamides and Unactivated Alkenes: Mechanistic Evidence for Vinyl C-H Bond Activation Promoted by an Electrophilic Ruthenium-Hydride Catalyst.Organometallics. 2010 Oct 21;29(22):5748-5750. doi: 10.1021/om100764c. Organometallics. 2010. PMID: 21344062 Free PMC article.
References
-
-
Reviews: Pillai SM, Ravindranathan M, Sivaram S. Chem. Rev. 1986;86:353–399.. Skupinska J. Chem. Rev. 1991;91:613–648.. Mecking S. Coord. Chem. Rev. 2000;203:325–351.. Speiser F, Braunstein P, Saussine L. Acc. Chem. Res. 2005;38:784–793..
-
-
- Olivier-Bourbigou H, Saussine L. In: Applied Homogeneous Catalysis with Organometallic Compounds. Cornils B, Herrmann WA, editors. Volume 1. Wiley-VCH; Weinheim: 2002.
- Keim W. Angew. Chem., Int. Ed. 1990;29:235–244.
-
- Keim W, Behr A, Röper M. In: Comprehensive Organometallic Chemistry. Wilkinson G, Stone FGA, Abel EW, editors. Volume 12. Pergamon Press; New York: 1982.
- Datta S, Fischer MB, Wreford SS. J. Organomet. Chem. 1980;188:353–366.
- Schrock R, McLain S, Sancho J. Pure Appl. Chem. 1980;52:729–732.
- Andes C, Harkins SB, Murtuza S, Oyler K, Sen A. J. Am. Chem. Soc. 2001;123:7423–7424. - PubMed
- Yu Z-X, Houk KN. Angew. Chem., Int. Ed. 2003;42:808–811. - PubMed
-
- Bhalla G, Oxgaard J, Goddard WA, III, Periana RA. Organometallics. 2005;24:5499–5502.
- Tsuchimoto T, Kamiyama S, Negoro R, Shirakawa E, Kawakami Y. Chem. Commun. 2003:852–853. - PubMed
-
-
Recent selected examples with Cr catalysts. Dixon JT, Green MJ, Hess FM, Morgan DH. J. Organomet. Chem. 2004;689:3641–3668.. McGuinness DS, Wasserscheid P, Keim W, Morgan D, Dixon JT, Bollmann A, Maumela H, Hess F, Englert U. J. Am. Chem. Soc. 2003;125:5272–5273.. Blann K, Bollmann A, Dixon JT, Hess FM, Killian E, Maumela H, Morgan DH, Neveling A, Otto S, Overett MJ. Chem. Commun. 2005:620–621.. Agapie T, Labinger JA, Bercaw JE. J. Am. Chem. Soc. 2007;129:14281–14295.. Arteaga-Müller R, Tsurugi H, Saito T, Yanagawa M, Oda S, Mashima K. J. Am. Chem. Soc. 2009;131:5370–5371..
-
Examples with Ti catalysts: Deckers PJW, Hessen B, Teuben JH. Angew. Chem., Int. Ed. 2001;40:2516–2519.. Otten E, Batinas AA, Meetsma A, Hessen B. J. Am. Chem. Soc. 2009;131:5298–5312.. Suzuki Y, Kinoshita S, Shibahara A, Ishii S, Kawamura K, Inoue Y, Fujita T. Organometallics. 2010;29:2394–2396..
-
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous