A Phase 1 study of UCN-01 in combination with irinotecan in patients with resistant solid tumor malignancies
- PMID: 20694727
- PMCID: PMC3102212
- DOI: 10.1007/s00280-010-1410-1
A Phase 1 study of UCN-01 in combination with irinotecan in patients with resistant solid tumor malignancies
Abstract
Purpose: UCN-01 (7-hydroxystaurosporine) is a multi-targeted protein kinase inhibitor that exhibits synergistic activity with DNA-damaging agents in preclinical studies. We conducted a Phase I study to determine the maximum-tolerated dose (MTD), dose-limiting toxicity (DLT), pharmacokinetic, and pharmacodynamic effects of UCN-01 and irinotecan in patients with resistant solid tumors.
Experimental design: Patients received irinotecan (75-125 mg/m(2) IV on days 1, 8, 15, 22) and UCN-01 (50-90 mg/m(2) IV on day 2 and 25-45 mg/m(2) on day 23 and subsequent doses) every 42 days. Blood for pharmacokinetics of UCN-01 and irinotecan, and blood, normal rectal mucosa, and tumor biopsies for pharmacodynamic studies were obtained.
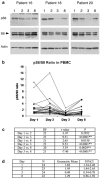
Results: Twenty-five patients enrolled to 5 dose levels. The MTD was irinotecan 125 mg/m(2) on days 1, 8, 15, 22 and UCN-01 70 mg/m(2) on day 2 and 35 mg/m(2) on day 23. DLTs included grade 3 diarrhea/dehydration and dyspnea. UCN-01 had a prolonged half-life and a low clearance rate. There was a significant reduction in SN-38 C(max) and aminopentanocarboxylic acid (APC) and SN-38 glucuronide half-lives. Phosphorylated ribosomal protein S6 was reduced in blood, normal rectal mucosa, and tumor biopsies at 24 h post-UCN-01. Two partial responses were observed in women with ER, PgR, and HER2-negative breast cancers (TBNC). Both tumors were defective for p53. Twelve patients had stable disease (mean duration 18 weeks, range 7-30 weeks).
Conclusion: UCN-01 and irinotecan demonstrated acceptable toxicity and target inhibition. Anti-tumor activity was observed and a study of this combination in women with TNBC is underway.
Figures

References
-
- Abramoff MD. Roaming through methodology XXIX. P. Ned Tijdschr Geneeskd. 2001;145:602. - PubMed
-
- Akiyama T, Shimizu M, Okabe M, Tamaoki T, Akinaga S. Differential effects of UCN-01, staurosporine and CGP 41 251 on cell cycle progression and CDC2/cyclin B1 regulation in A431 cells synchronized at M phase by nocodazole. Anticancer Drugs. 1999;10:67–78. doi: 10.1097/00001813-199901000-00009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

