Cellular communications in bone homeostasis and repair
- PMID: 20694737
- PMCID: PMC11115676
- DOI: 10.1007/s00018-010-0479-3
Cellular communications in bone homeostasis and repair
Abstract
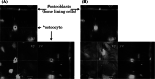
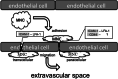
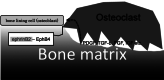
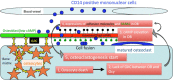
Cellular communication between the bone component cells osteoblasts, osteocytes and (pre-)osteoclasts is essential for bone remodeling which maintains bone integrity. As in the remodeling of other organs, cell death is a trigger for remodeling of bone. During the systematic process of bone remodeling, direct or indirect cell-cell communication is indispensable. Thus, osteoblasts induce migration and differentiation of preosteoclasts, which is followed by bone resorption (by mature multinuclear osteoclasts). After completion of bone resorption, apoptosis of mature osteoclasts and differentiation of osteoblasts are initiated. At this time, the osteoblasts do not support osteoclast differentiation but do support bone formation. Finally, osteoblasts differentiate to osteocytes in bone or to bone lining cells on bone surfaces. In this way, old bone areas are regenerated as new bone. In this review the role of cell-cell communication in bone remodeling is discussed.
Figures



References
-
- Burger EH, Klein-Nulend J. Mechanotransduction in bone – role of the lacuno-canalicular network. FASEB J. 1999;13(Suppl):S101–S112. - PubMed
-
- Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6:354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

