Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells
- PMID: 20694844
- PMCID: PMC3024091
- DOI: 10.1007/s12192-010-0216-8
Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells
Abstract
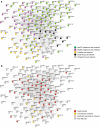
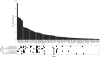
Molecular chaperones are central to cellular protein homeostasis. In mammals, protein misfolding diseases and aging cause inflammation and progressive tissue loss, in correlation with the accumulation of toxic protein aggregates and the defective expression of chaperone genes. Bacteria and non-diseased, non-aged eukaryotic cells effectively respond to heat shock by inducing the accumulation of heat-shock proteins (HSPs), many of which molecular chaperones involved in protein homeostasis, in reducing stress damages and promoting cellular recovery and thermotolerance. We performed a meta-analysis of published microarray data and compared expression profiles of HSP genes from mammalian and plant cells in response to heat or isothermal treatments with drugs. The differences and overlaps between HSP and chaperone genes were analyzed, and expression patterns were clustered and organized in a network. HSPs and chaperones only partly overlapped. Heat-shock induced a subset of chaperones primarily targeted to the cytoplasm and organelles but not to the endoplasmic reticulum, which organized into a network with a central core of Hsp90s, Hsp70s, and sHSPs. Heat was best mimicked by isothermal treatments with Hsp90 inhibitors, whereas less toxic drugs, some of which non-steroidal anti-inflammatory drugs, weakly expressed different subsets of Hsp chaperones. This type of analysis may uncover new HSP-inducing drugs to improve protein homeostasis in misfolding and aging diseases.
Figures
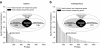



References
-
- Aalinkeel R, Bindukumar B, Reynolds JL, Sykes DE, Mahajan SD, Chadha KC, Schwartz SA. The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate. 2008;68(16):1773–1789. doi: 10.1002/pros.20845. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

