The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection
- PMID: 20696007
- PMCID: PMC6640454
- DOI: 10.1111/j.1364-3703.2010.00625.x
The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection
Abstract
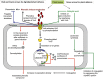
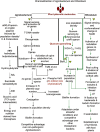
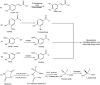
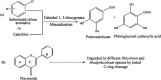
Phenolics are aromatic benzene ring compounds with one or more hydroxyl groups produced by plants mainly for protection against stress. The functions of phenolic compounds in plant physiology and interactions with biotic and abiotic environments are difficult to overestimate. Phenolics play important roles in plant development, particularly in lignin and pigment biosynthesis. They also provide structural integrity and scaffolding support to plants. Importantly, phenolic phytoalexins, secreted by wounded or otherwise perturbed plants, repel or kill many microorganisms, and some pathogens can counteract or nullify these defences or even subvert them to their own advantage. In this review, we discuss the roles of phenolics in the interactions of plants with Agrobacterium and Rhizobium.
Figures
References
-
- Adams, N.R. (1989) Phytoestrogens In: Toxicants of Plant Origin (Cheeke P.R., ed.), pp. 23–51. Boca Raton: CRC Press.
-
- Aguilar, J.M.M. , Ashby, A.M. , Richards, A.J.M. , Loake, G.J. , Watson, M.D. and Shaw, C.H. (1988) Chemotaxis of Rhizobium leguminosarum biovar phaseoli towards flavonoid inducers of the symbiotic nodulation genes. J. Gen. Microbiol. 134, 2741–2746.
-
- Akhtar, M. and Malik, A. (2000) Roles of organic soil amendments and soil organisms in the biological control of plant‐parasitic nematodes: a review. Bioresour. Technol. 74, 35–47.
-
- Akiyama, K. (2007) Chemical identification and functional analysis of apocarotenoids involved in the development of arbuscular mycorrhizal symbiosis. Biosci. Biotechnol. Biochem. 71, 1405–1414. - PubMed
-
- Akiyama, K. , Matsuzaki, K. and Hayashi, H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature, 435, 824–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

