Compliance assessment of ambulatory Alzheimer patients to aid therapeutic decisions by healthcare professionals
- PMID: 20696034
- PMCID: PMC2928215
- DOI: 10.1186/1472-6963-10-232
Compliance assessment of ambulatory Alzheimer patients to aid therapeutic decisions by healthcare professionals
Abstract
Background: Compliance represents a major determinant for the effectiveness of pharmacotherapy. Compliance reports summarising electronically compiled compliance data qualify healthcare needs and can be utilised as part of a compliance enhancing intervention. Nevertheless, evidence-based information on a sufficient level of compliance is scarce complicating the interpretation of compliance reports. The purpose of our pilot study was to determine the compliance of ambulatory Alzheimer patients to antidementia drugs under routine therapeutic use using electronic monitoring. In addition, the forgiveness of donepezil (i.e. its ability to sustain adequate pharmacological response despite suboptimal compliance) was characterised and evidence-based guidance for the interpretation of compliance reports was intended to be developed.
Methods: We determined the compliance of four different antidementia drugs by electronic monitoring in 31 patients over six months. All patients were recruited from the gerontopsychiatric clinic of a university hospital as part of a pilot study. The so called medication event monitoring system (MEMS) was employed, consisting of a vial with a microprocessor in the lid which records the time (date, hour, minute) of every opening. Daily compliance served as primary outcome measure, defined as percentage of days with correctly administered doses of medication. In addition, pharmacokinetics and pharmacodynamics of donepezil were simulated to systematically assess therapeutic undersupply also incorporating study compliance patterns. Statistical analyses were performed with SPSS and Microsoft Excel.
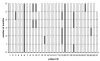
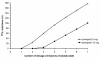
Results: Median daily compliance was 94% (range 48%-99%). Ten patients (32%) were non-compliant at least for one month. One-sixth of patients taking donepezil displayed periods of therapeutic undersupply. For 10 mg and 5 mg donepezil once-daily dosing, the estimated forgiveness of donepezil was 80% and 90% daily compliance or two and one dosage omissions at steady state, respectively. Based on the simulation findings we developed rules for the evidence-based interpretation of donepezil compliance reports.
Conclusions: Compliance in ambulatory Alzheimer patients was for the first time assessed under routine conditions using electronic monitoring: On average compliance was relatively high but variable between patients. The approach of pharmacokinetic/pharmacodynamic in silico simulations was suitable to characterise the forgiveness of donepezil suggesting evidence-based recommendations for the interpretation of compliance reports.
Figures


Similar articles
-
Efficacy and safety of donepezil in patients with Alzheimer's disease: results of a global, multinational, clinical experience study.Drugs Aging. 2004;21(1):43-53. doi: 10.2165/00002512-200421010-00004. Drugs Aging. 2004. PMID: 14715043
-
Another advertisement for donepezil.J Am Geriatr Soc. 2004 May;52(5):843; author reply 845-6. doi: 10.1111/j.1532-5415.2004.52230_1.x. J Am Geriatr Soc. 2004. PMID: 15086674 No abstract available.
-
Down syndrome and Alzheimer disease: response to donepezil.Arch Neurol. 2002 Jul;59(7):1133-6. doi: 10.1001/archneur.59.7.1133. Arch Neurol. 2002. PMID: 12117361 Clinical Trial.
-
Higher-dose (23 mg/day) donepezil formulation for the treatment of patients with moderate-to-severe Alzheimer's disease.Postgrad Med. 2012 Nov;124(6):110-6. doi: 10.3810/pgm.2012.11.2589. Postgrad Med. 2012. PMID: 23322144 Review.
-
Donepezil: a review.Expert Opin Drug Metab Toxicol. 2005 Oct;1(3):527-36. doi: 10.1517/17425255.1.3.527. Expert Opin Drug Metab Toxicol. 2005. PMID: 16863459 Review.
Cited by
-
Persistence and adherence with dementia pharmacotherapy: relevance of patient, provider, and system factors.Can J Psychiatry. 2014 Dec;59(12):624-31. doi: 10.1177/070674371405901203. Can J Psychiatry. 2014. PMID: 25702361 Free PMC article. Review.
-
An easy intervention to improve short-term adherence to medications in community-dwelling older outpatients. A pilot non-randomised controlled trial.BMC Health Serv Res. 2011 Jul 5;11:158. doi: 10.1186/1472-6963-11-158. BMC Health Serv Res. 2011. PMID: 21729274 Free PMC article.
-
Tolerability of Cholinesterase Inhibitors: A Population-Based Study of Persistence, Adherence, and Switching.Drugs Aging. 2017 Mar;34(3):221-231. doi: 10.1007/s40266-017-0438-x. Drugs Aging. 2017. PMID: 28138912
-
[Compliance enhancement in drug therapy : opportunities and limitations].Internist (Berl). 2012 Jan;53(1):99-107. doi: 10.1007/s00108-011-2951-z. Internist (Berl). 2012. PMID: 22119908 German.
-
Quantification of the Forgiveness of Drugs to Imperfect Adherence.CPT Pharmacometrics Syst Pharmacol. 2015 Mar;4(3):e00004. doi: 10.1002/psp4.4. Epub 2015 Mar 4. CPT Pharmacometrics Syst Pharmacol. 2015. PMID: 26225235 Free PMC article.
References
-
- AKdÄ. Arzneimittelkommission der deutschen Ärzteschaft - Therapieempfehlungen Demenz. 2008.
-
- GEK Arzneimittelverordnungsreport. 2009.
-
- Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

