Rapid modification of the insect elicitor N-linolenoyl-glutamate via a lipoxygenase-mediated mechanism on Nicotiana attenuata leaves
- PMID: 20696061
- PMCID: PMC3095298
- DOI: 10.1186/1471-2229-10-164
Rapid modification of the insect elicitor N-linolenoyl-glutamate via a lipoxygenase-mediated mechanism on Nicotiana attenuata leaves
Abstract
Background: Some plants distinguish mechanical wounding from herbivore attack by recognizing specific constituents of larval oral secretions (OS) which are introduced into plant wounds during feeding. Fatty acid-amino acid conjugates (FACs) are major constituents of Manduca sexta OS and strong elicitors of herbivore-induced defense responses in Nicotiana attenuata plants.
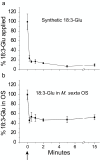
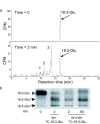
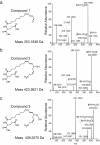
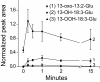
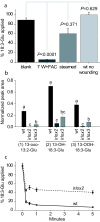
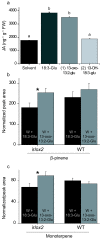
Results: The metabolism of one of the major FACs in M. sexta OS, N-linolenoyl-glutamic acid (18:3-Glu), was analyzed on N. attenuata wounded leaf surfaces. Between 50 to 70% of the 18:3-Glu in the OS or of synthetic 18:3-Glu were metabolized within 30 seconds of application to leaf wounds. This heat-labile process did not result in free alpha-linolenic acid (18:3) and glutamate but in the biogenesis of metabolites both more and less polar than 18:3-Glu. Identification of the major modified forms of this FAC showed that they corresponded to 13-hydroxy-18:3-Glu, 13-hydroperoxy-18:3-Glu and 13-oxo-13:2-Glu. The formation of these metabolites occurred on the wounded leaf surface and it was dependent on lipoxygenase (LOX) activity; plants silenced in the expression of NaLOX2 and NaLOX3 genes showed more than 50% reduced rates of 18:3-Glu conversion and accumulated smaller amounts of the oxygenated derivatives compared to wild-type plants. Similar to 18:3-Glu, 13-oxo-13:2-Glu activated the enhanced accumulation of jasmonic acid (JA) in N. attenuata leaves whereas 13-hydroxy-18:3-Glu did not. Moreover, compared to 18:3-Glu elicitation, 13-oxo-13:2-Glu induced the differential emission of two monoterpene volatiles (beta-pinene and an unidentified monoterpene) in irlox2 plants.
Conclusions: The metabolism of one of the major elicitors of herbivore-specific responses in N. attenuata plants, 18:3-Glu, results in the formation of oxidized forms of this FAC by a LOX-dependent mechanism. One of these derivatives, 13-oxo-13:2-Glu, is an active elicitor of JA biosynthesis and differential monoterpene emission.
Figures
Similar articles
-
Lipoxygenase-mediated modification of insect elicitors: generating chemical diversity on the leaf wound surface.Plant Signal Behav. 2010 Dec;5(12):1674-6. doi: 10.4161/psb.5.12.14036. Epub 2010 Dec 1. Plant Signal Behav. 2010. PMID: 21150262 Free PMC article.
-
Oxylipin channelling in Nicotiana attenuata: lipoxygenase 2 supplies substrates for green leaf volatile production.Plant Cell Environ. 2010 Dec;33(12):2028-40. doi: 10.1111/j.1365-3040.2010.02203.x. Plant Cell Environ. 2010. PMID: 20584148
-
Nicotiana attenuata SIPK, WIPK, NPR1, and fatty acid-amino acid conjugates participate in the induction of jasmonic acid biosynthesis by affecting early enzymatic steps in the pathway.Plant Physiol. 2010 Jan;152(1):96-106. doi: 10.1104/pp.109.149013. Epub 2009 Nov 6. Plant Physiol. 2010. PMID: 19897603 Free PMC article.
-
The essential role of jasmonic acid in plant-herbivore interactions--using the wild tobacco Nicotiana attenuata as a model.J Genet Genomics. 2013 Dec 20;40(12):597-606. doi: 10.1016/j.jgg.2013.10.001. Epub 2013 Nov 9. J Genet Genomics. 2013. PMID: 24377866 Review.
-
Glutamate: A multifunctional amino acid in plants.Plant Sci. 2022 May;318:111238. doi: 10.1016/j.plantsci.2022.111238. Epub 2022 Feb 24. Plant Sci. 2022. PMID: 35351313 Review.
Cited by
-
Recent advances in plant early signaling in response to herbivory.Int J Mol Sci. 2011;12(6):3723-39. doi: 10.3390/ijms12063723. Epub 2011 Jun 7. Int J Mol Sci. 2011. PMID: 21747702 Free PMC article. Review.
-
Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory.Plant Cell. 2011 Sep;23(9):3512-32. doi: 10.1105/tpc.111.088229. Epub 2011 Sep 16. Plant Cell. 2011. PMID: 21926334 Free PMC article.
-
Whiteflies glycosylate salicylic acid and secrete the conjugate via their honeydew.J Chem Ecol. 2015 Jan;41(1):52-8. doi: 10.1007/s10886-014-0543-9. Epub 2015 Jan 8. J Chem Ecol. 2015. PMID: 25563984 Free PMC article.
-
Tandem 13-Lipoxygenase Genes in a Cluster Confers Yellow-Green Leaf in Cucumber.Int J Mol Sci. 2019 Jun 25;20(12):3102. doi: 10.3390/ijms20123102. Int J Mol Sci. 2019. PMID: 31242619 Free PMC article.
-
The secret life of insect-associated microbes and how they shape insect-plant interactions.FEMS Microbiol Ecol. 2022 Aug 25;98(9):fiac083. doi: 10.1093/femsec/fiac083. FEMS Microbiol Ecol. 2022. PMID: 35830517 Free PMC article. Review.
References
-
- Labandeira C. The origin of herbivory on land: Initial patterns of plant tissue consumption by arthropods. Insect Sci. 2007;14:259–275. doi: 10.1111/j.1744-7917.2007.00152.x. - DOI
-
- Halitschke R, Gase K, Hui D, Schmidt DD, Baldwin IT. Molecular Interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol. 2003;131:1894–1902. doi: 10.1104/pp.102.018184. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

