Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants
- PMID: 20696166
- PMCID: PMC2998559
- DOI: 10.1016/j.febslet.2010.08.006
Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants
Abstract
The aggregation by antigen of the IgE bound to its high affinity receptor on mast cells initiates a complex series of biochemical events that result in the release of inflammatory mediators. The essential role of the protein tyrosine kinase Syk has been appreciated for some time, and newer results have defined the mechanism of its activation. The use of siRNA has defined the relative contribution of Syk, Fyn and Gab2 to signaling and has made possible a screening study to identify previously unrecognized molecules that are involved in these pathways.
Published by Elsevier B.V.
Figures

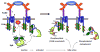


References
-
- Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–46. - PubMed
-
- Zhang J, Billingsley ML, Kincaid RL, Siraganian RP. Phosphorylation of Syk activation loop tyrosines is essential for Syk function. An in vivo study using a specific anti-Syk activation loop phosphotyrosine antibody. J Biol Chem. 2000;275:35442–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

