Human endogenous retroviruses and multiple sclerosis: innocent bystanders or disease determinants?
- PMID: 20696240
- PMCID: PMC7172332
- DOI: 10.1016/j.bbadis.2010.07.016
Human endogenous retroviruses and multiple sclerosis: innocent bystanders or disease determinants?
Abstract
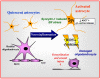
Human endogenous retroviruses (HERVs) constitute 5-8% of human genomic DNA and are replication incompetent despite expression of individual HERV genes from different chromosomal loci depending on the specific tissue. Several HERV genes have been detected as transcripts and proteins in the central nervous system, frequently in the context of neuroinflammation. The HERV-W family has received substantial attention in large part because of associations with diverse syndromes including multiple sclerosis (MS) and several psychiatric disorders. A HERV-W-related retroelement, multiple sclerosis retrovirus (MSRV), has been reported in MS patients to be both a biomarker as well as an effector of aberrant immune responses. HERV-H and HERV-K have also been implicated in MS and other neurological diseases but await delineation of their contributions to disease. The HERV-W envelope-encoded glycosylated protein, syncytin-1, is encoded by chromosome 7q21 and exhibits increased glial expression within MS lesions. Overexpression of syncytin-1 in glia induces endoplasmic reticulum stress leading to neuroinflammation and the induction of free radicals, which damage proximate cells. Syncytin-1's receptor, ASCT1 is a neutral amino acid transporter expressed on glia and is suppressed in white matter of MS patients. Of interest, antioxidants ameliorate syncytin-1's neuropathogenic effects raising the possibility of using these agents as therapeutics for neuroinflammatory diseases. Given the multiple insertion sites of HERV genes as complete and incomplete open reading frames, together with their differing capacity to be expressed and the complexities of individual HERVs as both disease markers and bioactive effectors, HERV biology is a compelling area for understanding neuropathogenic mechanisms and developing new therapeutic strategies.
2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Sospedra M., Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. - PubMed
-
- Power C. Retroviral diseases of the nervous system: pathogenic host response or viral gene-mediated neurovirulence? Trends Neurosci. 2001;24(3):162–169. - PubMed
-
- Hughes J.F., Coffin J.M. Evidence for genomic rearrangements mediated by human endogenous retroviruses during primate evolution. Nat. Genet. 2001;29(4):487–489. - PubMed
-
- Costas J. Characterization of the intragenomic spread of the human endogenous retrovirus family HERV-W. Mol. Biol. Evol. 2002;19(4):526–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

