Overexpression of glia maturation factor reinstates susceptibility to myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis in glia maturation factor deficient mice
- PMID: 20696246
- PMCID: PMC2955779
- DOI: 10.1016/j.nbd.2010.08.003
Overexpression of glia maturation factor reinstates susceptibility to myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis in glia maturation factor deficient mice
Abstract
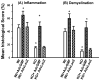
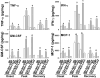
Glia maturation factor (GMF), a primarily CNS localized protein was discovered and characterized in our laboratory. We previously demonstrated that GMF is the upstream regulator for excessive production and release of proinflammatory cytokines/chemokines in brain cells leading to the destruction of oligodendrocytes, the myelin forming cells, and neurons. We also reported that mice lacking endogenous GMF (GMF-deficient, GMF-KO) were resistant to myelin oligodendrocyte glycoprotein peptide 35-55 (MOG(35-55)) induced EAE, since immunization induced only delayed EAE with diminished severity. In the present study we show that a replication-defective adenovirus-GMF construct caused expression of GMF in CNS of GMF-KO mice and reinstated MOG(35-55) induced early and severe EAE. Our results show that MOG(35-55) immunization caused only a muted EAE and inflammation/demyelination in mice lacking endogenous GMF. The diminished incidence of EAE in GMF-KO mice was consistent with the significantly reduced expressions of cytokines/chemokines. The muted severity of EAE in GMF-KO mice was restored to full blown levels upon reintroduction of GMF using an adeno-GMF-virus (Adv-GMF) vector. Consistent with the clinical findings, histological examination of the CNS of mice with EAE revealed profound differences between wild type (Wt), GMF-KO, and GMF-KO mice with re-introduced GMF (GMF-KO+Adv-GMF). Spinal cord sections from mice with EAE were analyzed for the infiltration of mononuclear cells (inflammation) and myelin loss (demyelination). In Wt mice, 40% of spinal cord quadrants were positive for demyelination and 45% of spinal cord quadrants were positive for inflammation at the peak of EAE. Drastically reduced infiltrates (15%) and demyelination (10%) were found in GMF-KO mice that developed reduced severity of EAE. Upon GMF reintroduction in GMF-KO mice, MOG(35-55) immunization caused extensive monocytes infiltration (48%) and demyelination (46%), similar to that observed in the immunized Wt mice. The levels of cytokine/chemokine in the spinal cords of mice at three time points, corresponding to the onset, peak severity and recovery period of EAE, show a distinct pattern of very large increases in IFN-γ, TNF-α, GM-CSF and MCP-1 in Wt and GMF-KO+Adv-GMF mice compared to GMF-KO and GMF-KO+Adv-LacZ mice.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Suppression of neuro inflammation in experimental autoimmune encephalomyelitis by glia maturation factor antibody.Brain Res. 2011 Feb 10;1373:230-9. doi: 10.1016/j.brainres.2010.12.003. Epub 2010 Dec 10. Brain Res. 2011. PMID: 21146509 Free PMC article.
-
Clinical course of myelin oligodendrocyte glycoprotein 35-55 induced experimental autoimmune encephalomyelitis is aggravated by glia maturation factor.Neurochem Int. 2012 Feb;60(3):215-9. doi: 10.1016/j.neuint.2011.12.011. Epub 2011 Dec 30. Neurochem Int. 2012. PMID: 22226840 Free PMC article.
-
Diminished cytokine and chemokine expression in the central nervous system of GMF-deficient mice with experimental autoimmune encephalomyelitis.Brain Res. 2007 May 4;1144:239-47. doi: 10.1016/j.brainres.2007.01.075. Epub 2007 Jan 27. Brain Res. 2007. PMID: 17316572 Free PMC article.
-
Glia maturation factor regulation of STAT expression: a novel mechanism in experimental autoimmune encephalomyelitis.Neurochem Res. 2007 Dec;32(12):2123-31. doi: 10.1007/s11064-007-9383-0. Epub 2007 Jun 6. Neurochem Res. 2007. PMID: 17551829
-
VPAC1 receptor (Vipr1)-deficient mice exhibit ameliorated experimental autoimmune encephalomyelitis, with specific deficits in the effector stage.J Neuroinflammation. 2016 Jun 29;13(1):169. doi: 10.1186/s12974-016-0626-3. J Neuroinflammation. 2016. PMID: 27357191 Free PMC article.
Cited by
-
Cellular and pathophysiological consequences of Arp2/3 complex inhibition: role of inhibitory proteins and pharmacological compounds.Cell Mol Life Sci. 2019 Sep;76(17):3349-3361. doi: 10.1007/s00018-019-03128-y. Epub 2019 May 9. Cell Mol Life Sci. 2019. PMID: 31073744 Free PMC article. Review.
-
First description of enhanced expression of glia maturation factor-beta in experimental toxoplasmic encephalitis.J Int Med Res. 2017 Dec;45(6):1670-1679. doi: 10.1177/0300060517700320. Epub 2017 Aug 4. J Int Med Res. 2017. PMID: 28774213 Free PMC article.
-
Mast Cells Release Chemokine CCL2 in Response to Parkinsonian Toxin 1-Methyl-4-Phenyl-Pyridinium (MPP(+)).Neurochem Res. 2016 May;41(5):1042-9. doi: 10.1007/s11064-015-1790-z. Epub 2015 Dec 8. Neurochem Res. 2016. PMID: 26646004 Free PMC article.
-
Suppression of neuro inflammation in experimental autoimmune encephalomyelitis by glia maturation factor antibody.Brain Res. 2011 Feb 10;1373:230-9. doi: 10.1016/j.brainres.2010.12.003. Epub 2010 Dec 10. Brain Res. 2011. PMID: 21146509 Free PMC article.
-
Enhanced expression of glia maturation factor correlates with glial activation in the brain of triple transgenic Alzheimer's disease mice.Neurochem Res. 2013 Jan;38(1):218-25. doi: 10.1007/s11064-012-0913-z. Epub 2012 Oct 20. Neurochem Res. 2013. PMID: 23086473 Free PMC article.
References
-
- Akkad DA, et al. Variation in the IL7RA and IL2RA genes in German multiple sclerosis patients. J Autoimmun. 2009;32:110–115. - PubMed
-
- Al-Omaishi J, et al. The cellular immunology of multiple sclerosis. J Leukoc Biol. 1999;65:444–452. - PubMed
-
- Bright JJ, et al. Prevention of experimental allergic encephalomyelitis via inhibition of IL-12 signaling and IL-12-mediated Th1 differentiation: an effect of the novel anti-inflammatory drug lisofylline. J Immunol. 1998;161:7015–7022. - PubMed
-
- Bright JJ, et al. Tyrphostin B42 inhibits IL-12-induced tyrosine phosphorylation and activation of Janus kinase-2 and prevents experimental allergic encephalomyelitis. J Immunol. 1999a;162:6255–6262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

