Manifestation of ocular-muscle EMG contamination in human intracranial recordings
- PMID: 20696256
- PMCID: PMC2975438
- DOI: 10.1016/j.neuroimage.2010.08.002
Manifestation of ocular-muscle EMG contamination in human intracranial recordings
Abstract
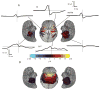
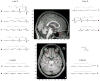
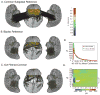
It is widely assumed that intracranial recordings from the brain are only minimally affected by contamination due to ocular-muscle electromyogram (oEMG). Here we show that this is not always the case. In intracranial recordings from five surgical epilepsy patients we observed that eye movements caused a transient biphasic potential at the onset of a saccade, resembling the saccadic spike potential commonly seen in scalp EEG, accompanied by an increase in broadband power between 20 and 200 Hz. Using concurrently recorded eye movements and high-density intracranial EEG (iEEG) we developed a detailed overview of the spatial distribution and temporal characteristics of the saccade-related oculomotor signal within recordings from ventral, medial and lateral temporal cortex. The occurrence of the saccadic spike was not explained solely by reference contact location, and was observed near the temporal pole for small (<2 deg) amplitude saccades and over a broad area for larger saccades. We further examined the influence of saccade-related oEMG contamination on measurements of spectral power and interchannel coherence. Contamination manifested in both spectral power and coherence measurements, in particular, over the anterior half of the ventral and medial temporal lobe. Next, we compared methods for removing the contaminating signal and found that nearest-neighbor bipolar re-referencing and ICA filtering were effective for suppressing oEMG at locations far from the orbits, but tended to leave some residual contamination at the temporal pole. Finally, we show that genuine cortical broadband gamma responses observed in averaged data from ventral temporal cortex can bear a striking similarity in time course and band-width to oEMG contamination recorded at more anterior locations. We conclude that eye movement-related contamination should be ruled out when reporting high gamma responses in human intracranial recordings, especially those obtained near anterior and medial temporal lobe.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures












References
-
- Akaike H. A new look at the statistical model identification. Automatic Control, IEEE Transactions on. 1974;19:716–723.
-
- Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–430. - PubMed
-
- Baillet S, Mosher JC, Leahy RM. Electromagnetic brain mapping. Signal Processing Magazine, IEEE. 2001;18:14–30.
-
- Ball T, Kern M, Mutschler I, Aertsen A, Schulze-Bonhage A. Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage. 2009;46:708–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

