DNA polymerase structure-based insight on the mutagenic properties of 8-oxoguanine
- PMID: 20696268
- PMCID: PMC3023916
- DOI: 10.1016/j.mrgentox.2010.07.013
DNA polymerase structure-based insight on the mutagenic properties of 8-oxoguanine
Abstract
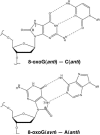
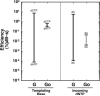
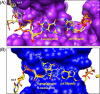
An aerobic environment burdens DNA polymerase substrates with oxidized substrates (DNA and nucleotide pools). A major promutagenic lesion resulting from oxidative stress is 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxoG). Guanine oxidation alters the hydrogen bonding properties of the base and glycosidic-preference of the nucleotide. The favored glycosidic syn-conformation exposes the Hoogsteen edge of the base for hydrogen bonding with adenine during DNA synthesis. The cell has recognized the threat of this lesion and has evolved an intricate surveillance system to provide DNA polymerases with unmodified substrates. Failure to do so leads to transversion mutations. Since the mutagenic properties of the base are dictated by the anti-syn-conformation of the nucleotide, the molecular interactions of 8-oxoG in the confines of the DNA polymerase active site are expected to influence its coding potential. Recent structural characterization of DNA polymerases from several families with this lesion in the nascent base pair binding pocket has provided insight to the mutagenic properties of this modified nucleotide. These structures reveal that flexibility around the template-binding pocket can permit 8-oxoG to assume an anti- or syn-conformation and code for cytosine or adenine incorporation, respectively. In contrast, the binding pocket for the incoming nucleotide does not have this flexibility so that 8-oxodGTP insertion opposite cytosine is strongly discouraged.
Published by Elsevier B.V.
Figures


References
-
- Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat. Res. 1991;250:3–16. - PubMed
-
- Boiteux S, Le Page F. Repair of 8-oxoguanine and Ogg1-incised apurinic sites in a CHO cell line. Prog. Nucleic Acid Res. Mol. Biol. 2001;68:95–105. - PubMed
-
- Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: An enemy within. Trends Genet. 1993;9:246–249. - PubMed
-
- Nakabeppu Y. Regulation of intracellular localization of human MTH1, OGG1, and MYH proteins for repair of oxidative DNA damage. Prog. Nucleic Acid Res. Mol. Biol. 2001;68:75–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

