Pathophysiological roles for purines: adenosine, caffeine and urate
- PMID: 20696321
- PMCID: PMC3102301
- DOI: 10.1016/S0079-6123(10)83010-9
Pathophysiological roles for purines: adenosine, caffeine and urate
Abstract
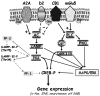
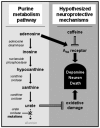
The motor symptoms of Parkinson's disease (PD) are primarily due to the degeneration of the dopaminergic neurons in the nigrostriatal pathway. However, several other brain areas and neurotransmitters other than dopamine such as noradrenaline, 5-hydroxytryptamine and acetylcholine are affected in the disease. Moreover, adenosine because of the extensive interaction of its receptors with the dopaminergic system has been implicated in the pathophysiology of the disease. Based on the involvement of these non-dopaminergic neurotransmitters in PD and the sometimes severe adverse effects that limit the mainstay use of dopamine-based anti-parkinsonian treatments, recent assessments have called for a broadening of therapeutic options beyond the traditional dopaminergic drug arsenal. In this review we describe the interactions between dopamine and adenosine receptors that underpin the pre-clinical and clinical rationale for pursuing adenosine A(2A) receptor antagonists as symptomatic and potentially neuroprotective treatment of PD. The review will pay particular attention to recent results regarding specific A(2A) receptor-receptor interactions and recent findings identifying urate, the end product of purine metabolism, as a novel prognostic biomarker and candidate neuroprotectant in PD.
2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55(no. 3):509–560. - PubMed
-
- Alfinito PD, Wang SP, Manzino L, Rijhsinghani S, Zeevalk GD, Sonsalla PK. Adenosinergic protection of dopaminergic and GABAergic neurons against mitochondrial inhibition through receptors located in the substantia nigra and striatum, respectively. J Neurosci. 2003;23(no. 4):10982–10987. - PMC - PubMed
-
- Alonso A, Rodríguez LA, Logroscino G, Hernán MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69:1696–1700. - PubMed
-
- Andreadou E, Nikolaou C, Gournaras F, Rentzos M, Boufidou F, Tsoutsou A, Zournas C, Zissimopoulos V, Vassilopoulos D. Serum uric acid levels in patients with Parkinson's disease: their relationship to treatment and disease duration. Clin Neurol Neurosurg. 2009;111(no. 9):724–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

