Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer's disease
- PMID: 20696495
- PMCID: PMC2980562
- DOI: 10.1016/j.neurobiolaging.2010.06.014
Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer's disease
Abstract
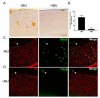
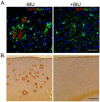
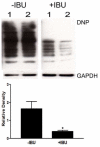
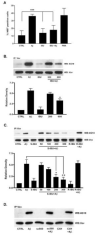
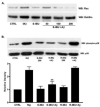
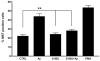
Considerable evidence points to important roles for inflammation in Alzheimer's disease (AD) pathophysiology. Epidemiological studies have suggested that long-term nonsteroidal anti-inflammatory drug (NSAID) therapy reduces the risk for Alzheimer's disease; however, the mechanism remains unknown. We report that a 9-month treatment of aged R1.40 mice resulted in 90% decrease in plaque burden and a similar reduction in microglial activation. Ibuprofen treatment reduced levels of lipid peroxidation, tyrosine nitration, and protein oxidation, demonstrating a dramatic effect on oxidative damage in vivo. Fibrillar β-amyloid (Aβ) stimulation has previously been demonstrated to induce the assembly and activation of the microglial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase leading to superoxide production through a tyrosine kinase-based signaling cascade. Ibuprofen treatment of microglia or monocytes with racemic or S-ibuprofen inhibited Aβ-stimulated Vav tyrosine phosphorylation, NADPH oxidase assembly, and superoxide production. Interestingly, Aβ-stimulated Vav phosphorylation was not inhibited by COX inhibitors. These findings suggest that ibuprofen acts independently of cyclooxygenase COX inhibition to disrupt signaling cascades leading to microglial NADPH oxidase (NOX2) activation, preventing oxidative damage and enhancing plaque clearance in the brain.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
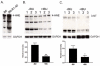
References
-
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama. 2003;289(21):2819–26. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. - PMC - PubMed
-
- Arvanitakis Z, Grodstein F, Bienias JL, Schneider JA, Wilson RS, Kelly JF, Evans DA, Bennett DA. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology. 2008;70(23):2219–25. - PubMed
-
- Asanuma M, Nishibayashi-Asanuma S, Miyazaki I, Kohno M, Ogawa N. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J Neurochem. 2001;76(6):1895–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

