Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund's adjuvant-induced inflammatory pain but not for incision-induced post-operative pain
- PMID: 20696523
- PMCID: PMC2939307
- DOI: 10.1016/j.pain.2010.07.017
Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund's adjuvant-induced inflammatory pain but not for incision-induced post-operative pain
Abstract
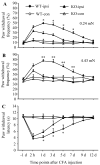
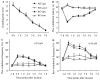
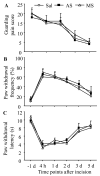
Protein interacting with C kinase 1 (PICK1) is a PDZ-containing protein that binds to AMPA receptor (AMPAR) GluR2 subunit and protein kinase Cα (PKCα) in the central neurons. It functions as a targeting and transport protein, presents the activated form of PKCα to synaptic GluR2, and participates in synaptic AMPAR trafficking in the nervous system. Thus, PICK1 might be involved in many physiological and pathological processes triggered via the activation of AMPARs. We report herein that PICK1 knockout mice display impaired mechanical and thermal pain hypersensitivities during complete Freund's adjuvant (CFA)-induced inflammatory pain maintenance. Acute transient knockdown of spinal cord PICK1 through intrathecal injection of PICK1 antisense oligodeoxynucleotide had a similar effect. In contrast, knockout and knockdown of spinal cord PICK1 did not affect incision-induced guarding pain behaviors or mechanical or thermal pain hypersensitivities. We also found that PICK1 is highly expressed in dorsal horn, where it interacts with GluR2 and PKCα. Injection of CFA into a hind paw, but not a hind paw incision, increased PKCα-mediated GluR2 phosphorylation at Ser880 and GluR2 internalization in dorsal horn. These increases were absent when spinal cord PICK1 was deficient. Given that dorsal horn PKCα-mediated GluR2 phosphorylation at Ser880 and GluR2 internalization contribute to the maintenance of CFA-induced inflammatory pain, our findings suggest that spinal PICK1 may participate in the maintenance of persistent inflammatory pain, but not in incision-induced post-operative pain, through promoting PKCα-mediated GluR2 phosphorylation and internalization in dorsal horn neurons.
Copyright © 2010 International Association for the Study of Pain. All rights reserved.
Figures




Similar articles
-
Islet-cell autoantigen 69 mediates the antihyperalgesic effects of electroacupuncture on inflammatory pain by regulating spinal glutamate receptor subunit 2 phosphorylation through protein interacting with C-kinase 1 in mice.Pain. 2019 Mar;160(3):712-723. doi: 10.1097/j.pain.0000000000001450. Pain. 2019. PMID: 30699097 Free PMC article.
-
PICK1 Regulates the Expression and Trafficking of AMPA Receptors in Remifentanil-Induced Hyperalgesia.Anesth Analg. 2016 Sep;123(3):771-81. doi: 10.1213/ANE.0000000000001442. Anesth Analg. 2016. PMID: 27537764
-
PKCα is required for inflammation-induced trafficking of extrasynaptic AMPA receptors in tonically firing lamina II dorsal horn neurons during the maintenance of persistent inflammatory pain.J Pain. 2013 Feb;14(2):182-92. doi: 10.1016/j.jpain.2012.10.015. J Pain. 2013. PMID: 23374940 Free PMC article.
-
Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain.Anesthesiology. 2010 May;112(5):1259-65. doi: 10.1097/ALN.0b013e3181d3e1ed. Anesthesiology. 2010. PMID: 20395828 Free PMC article. Review.
-
The Scaffold Protein PICK1 as a Target in Chronic Pain.Cells. 2022 Apr 7;11(8):1255. doi: 10.3390/cells11081255. Cells. 2022. PMID: 35455935 Free PMC article. Review.
Cited by
-
Role for neonatal D-serine signaling: prevention of physiological and behavioral deficits in adult Pick1 knockout mice.Mol Psychiatry. 2016 Mar;21(3):386-93. doi: 10.1038/mp.2015.61. Epub 2015 May 26. Mol Psychiatry. 2016. PMID: 26008737 Free PMC article.
-
Opioid receptors inhibit the spinal AMPA receptor Ca2+ permeability that mediates latent pain sensitization.Exp Neurol. 2019 Apr;314:58-66. doi: 10.1016/j.expneurol.2019.01.003. Epub 2019 Jan 17. Exp Neurol. 2019. PMID: 30660616 Free PMC article.
-
Role of dorsal root ganglion K2p1.1 in peripheral nerve injury-induced neuropathic pain.Mol Pain. 2017 Jan;13:1744806917701135. doi: 10.1177/1744806917701135. Mol Pain. 2017. PMID: 28326939 Free PMC article.
-
AMPA receptor trafficking in inflammation-induced dorsal horn central sensitization.Neurosci Bull. 2012 Apr;28(2):111-20. doi: 10.1007/s12264-012-1204-z. Neurosci Bull. 2012. PMID: 22466122 Free PMC article. Review.
-
Mechanisms of acupuncture-electroacupuncture on inflammatory pain.Mol Pain. 2023 Jan-Dec;19:17448069231202882. doi: 10.1177/17448069231202882. Mol Pain. 2023. PMID: 37678839 Free PMC article. Review.
References
-
- Banik RK, Woo YC, Park SS, Brennan TJ. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006;105:1246–53. - PubMed
-
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–98. - PubMed
-
- Chu YC, Chan KH, Tsou MY, Lin SM, Hsieh YC, Tao YX. Mechanical pain hypersensitivity after incisional surgery is enhanced in rats subjected to neonatal peripheral inflammation: effects of N-methyl-D-aspartate receptor antagonists. Anesthesiology. 2007;106:1204–12. - PubMed
-
- Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund's adjuvant-induced persistent pain. Pain. 2005;119:113–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

