Mechanism of regulation of native cardiac muscle thin filaments by rigor cardiac myosin-S1 and calcium
- PMID: 20696756
- PMCID: PMC2963418
- DOI: 10.1074/jbc.M109.098228
Mechanism of regulation of native cardiac muscle thin filaments by rigor cardiac myosin-S1 and calcium
Abstract
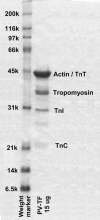
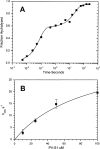
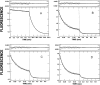
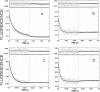
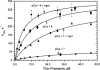
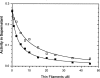
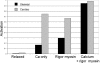
We have studied the mechanism of activation of native cardiac thin filaments by calcium and rigor myosin. The acceleration of the rate of 2'-deoxy-3'-O-(N-methylanthraniloyl)ADP (mdADP) dissociation from cardiac myosin-S1-mdADP-P(i) and cardiac myosin-S1-mdADP by native cardiac muscle thin filaments was measured using double mixing stopped-flow fluorescence. Relative to inhibited thin filaments (no bound calcium or rigor S1), fully activated thin filaments (with both calcium and rigor-S1 bound) increase the rate of product dissociation from the physiologically important pre-power stroke myosin-mdADP-P(i) by a factor of ∼75. This can be compared with only an ∼6-fold increase in the rate of nucleotide diphosphate dissociation from nonphysiological myosin-mdADP by the fully activated thin filaments relative to the fully inhibited thin filaments. These results show that physiological levels of regulation are not only dependent on the state of the thin filament but also on the conformation of the myosin. Less than 2-fold regulation is due to a change in affinity of myosin-ADP-P(i) for thin filaments such as would be expected by a simple "steric blocking" of the myosin-binding site of the thin filament by tropomyosin. Although maximal activation requires both calcium and rigor myosin-S1 bound to the cardiac filament, association with a single ligand produces ∼70% maximal activation. This can be contrasted with skeletal thin filaments in which calcium alone only activated the rate of product dissociation ∼20% of maximum, and rigor myosin produces ∼30% maximal activation.
Figures

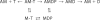
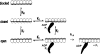
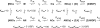
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

