A new Kv1.2 channelopathy underlying cerebellar ataxia
- PMID: 20696761
- PMCID: PMC2952217
- DOI: 10.1074/jbc.M110.153676
A new Kv1.2 channelopathy underlying cerebellar ataxia
Abstract
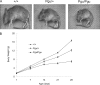
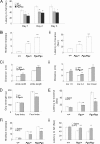
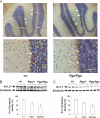
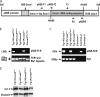
A forward genetic screen of mice treated with the mutagen ENU identified a mutant mouse with chronic motor incoordination. This mutant, named Pingu (Pgu), carries a missense mutation, an I402T substitution in the S6 segment of the voltage-gated potassium channel Kcna2. The gene Kcna2 encodes the voltage-gated potassium channel α-subunit Kv1.2, which is abundantly expressed in the large axon terminals of basket cells that make powerful axo-somatic synapses onto Purkinje cells. Patch clamp recordings from cerebellar slices revealed an increased frequency and amplitude of spontaneous GABAergic inhibitory postsynaptic currents and reduced action potential firing frequency in Purkinje cells, suggesting that an increase in GABA release from basket cells is involved in the motor incoordination in Pgu mice. In line with immunochemical analyses showing a significant reduction in the expression of Kv1 channels in the basket cell terminals of Pgu mice, expression of homomeric and heteromeric channels containing the Kv1.2(I402T) α-subunit in cultured CHO cells revealed subtle changes in biophysical properties but a dramatic decrease in the amount of functional Kv1 channels. Pharmacological treatment with acetazolamide or transgenic complementation with wild-type Kcna2 cDNA partially rescued the motor incoordination in Pgu mice. These results suggest that independent of known mutations in Kcna1 encoding Kv1.1, Kcna2 mutations may be important molecular correlates underlying human cerebellar ataxic disease.
Figures



References
-
- Hille B. (2001) Ionic Channels of Excitable Membranes, Sinauer, Sunderland, MA
-
- Hwang P. M., Glatt C. E., Bredt D. S., Yellen G., Snyder S. H. (1992) Neuron 8, 473–481 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

